Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study
Abstract
:1. Introduction
2. Methods
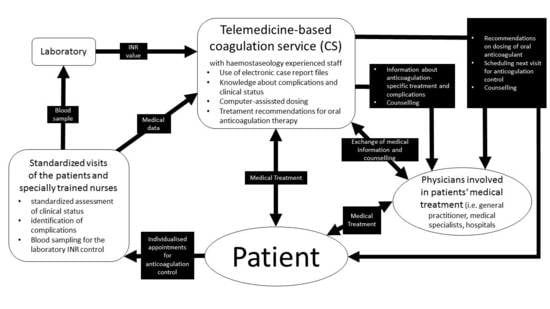
2.1. Study Design
2.2. Assessment of Study Data
2.3. Definition of Outcome Parameters
2.4. Statistical Analysis
3. Results
3.1. Analysis of Key Data and Patient Characteristics
3.2. Quality of Anticoagulation Therapy
3.3. Comparison of Clinical Outcome between Regular Medical Care and Coagulation Service
3.4. Subgroup Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCP | American College of Clinical Pharmacy |
| AF | Atrial fibrillation |
| ATE | Average treatment effect |
| CAD | Coronary artery disease |
| CATI | Computer-assisted telephone interview |
| CI | Confidence interval |
| CS | Coagulation service |
| DVT | Deep venous thrombosis |
| HR | Hazard ratio |
| INR | International Normalized Ratio |
| IQR | Inter-quartile range |
| MI | Myocardial infarction |
| DOAC | Direct-acting (non-vitamin K Antagonist) oral anticoagulants |
| OAC | Oral anticoagulation |
| OR | Odds ratio |
| PE | Pulmonary embolism |
| RMC | Regular medical care |
| RR | Rate ratio = Risk ratio |
| SD | Standard deviation |
| SMR | Standardized mortality ratio |
| TIA | Transient ischemic attack |
| TR | Therapeutic range |
| TTR | Time in therapeutic range |
| VKA | Vitamin K Antagonist |
| VTE | Venous thromboembolism |
References
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.P.; Munzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinides, S.V.; Meyer, G. The 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2019, 40, 3453–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, A.E.; Mahan, C.E.; Vazquez, S.R.; Oertel, L.B.; Garcia, D.A.; Ansell, J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J. Thromb. Thrombolysis 2016, 41, 206–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Rahman, A.; Carrier, M.; Kearon, C.; Weitz, J.I.; Schulman, S.; Couturaud, F.; Eichinger, S.; Kyrle, P.A.; Becattini, C.; et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: Systematic review and meta-analysis. BMJ 2019, 366, l4363. [Google Scholar] [CrossRef] [Green Version]
- Witt, D.M.; Delate, T.; Clark, N.P.; Martell, C.; Tran, T.; Crowther, M.A.; Garcia, D.A.; Ageno, W.; Hylek, E.M. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood 2009, 114, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; Raskob, G.E.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef] [Green Version]
- Kearon, C.; Gent, M.; Hirsh, J.; Weitz, J.; Kovacs, M.J.; Anderson, D.R.; Turpie, A.G.; Green, D.; Ginsberg, J.S.; Wells, P.; et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N. Engl. J. Med. 1999, 340, 901–907. [Google Scholar] [CrossRef]
- Almutairi, A.R.; Zhou, L.; Gellad, W.F.; Lee, J.K.; Slack, M.K.; Martin, J.R.; Lo-Ciganic, W.H. Effectiveness and Safety of Non-vitamin K Antagonist Oral Anticoagulants for Atrial Fibrillation and Venous Thromboembolism: A Systematic Review and Meta-analyses. Clin. Ther. 2017, 39, 1456–1478 e1436. [Google Scholar] [CrossRef]
- Becattini, C.; Vedovati, M.C.; Agnelli, G. Old and new oral anticoagulants for venous thromboembolism and atrial fibrillation: A review of the literature. Thromb. Res. 2012, 129, 392–400. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Schellong, S.; Eriksson, H.; Baanstra, D.; Kvamme, A.M.; Friedman, J.; Mismetti, P.; Goldhaber, S.Z.; et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N. Engl. J. Med. 2013, 368, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Sindet-Pedersen, C.; Pallisgaard, J.L.; Staerk, L.; Berger, J.S.; Lamberts, M.; Torp-Pedersen, C.; Gislason, G.H.; Olesen, J.B. Temporal trends in initiation of VKA, rivaroxaban, apixaban and dabigatran for the treatment of venous thromboembolism—A Danish nationwide cohort study. Sci. Rep. 2017, 7, 3347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prochaska, J.H.; Gobel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Junger, C.; Al-Bayati, Z.; Baer, C.; Walter, U.; et al. Quality of oral anticoagulation with phenprocoumon in regular medical care and its potential for improvement in a telemedicine-based coagulation service inverted question mark results from the prospective, multi-center, observational cohort study thrombEVAL. BMC Med. 2015, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.J.; Pogue, J.; Eikelboom, J.; Flaker, G.; Commerford, P.; Franzosi, M.G.; Healey, J.S.; Yusuf, S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008, 118, 2029–2037. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Heneghan, C.; Perera, R.; Roberts, N.; Hollowell, J.; Glasziou, P.; Bankhead, C.; Xu, Y. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2008, 1, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Rosendaal, F.R.; Cannegieter, S.C.; van der Meer, F.J.; Briet, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 1993, 69, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galie, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069, 3069a–3069k. [Google Scholar] [CrossRef] [Green Version]
- Konstantinides, S.V.; Barco, S.; Lankeit, M.; Meyer, G. Management of Pulmonary Embolism: An Update. J. Am. Coll. Cardiol. 2016, 67, 976–990. [Google Scholar] [CrossRef]
- van Walraven, C.; Jennings, A.; Oake, N.; Fergusson, D.; Forster, A.J. Effect of study setting on anticoagulation control: A systematic review and metaregression. Chest 2006, 129, 1155–1166. [Google Scholar] [CrossRef]
- Chiquette, E.; Amato, M.G.; Bussey, H.I. Comparison of an anticoagulation clinic with usual medical care: Anticoagulation control, patient outcomes, and health care costs. Arch. Intern. Med. 1998, 158, 1641–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, D.M.; Sadler, M.A.; Shanahan, R.L.; Mazzoli, G.; Tillman, D.J. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest 2005, 127, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.M.; Dier, J.G. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy 2010, 30, 330–338. [Google Scholar] [CrossRef]
- Barnes, G.D.; Nallamothu, B.K.; Sales, A.E.; Froehlich, J.B. Reimagining Anticoagulation Clinics in the Era of Direct Oral Anticoagulants. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 182–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.H.; Akl, E.A.; Crowther, M.; Gutterman, D.D.; Schuunemann, H.J. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, S7–S47. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Coldewey, M.; Gobel, S.; Keller, K.; Hendelmeier, M.; Konstantinides, S.; Munzel, T.; Wild, P.S. Evaluation of oral anticoagulation therapy: Rationale and design of the thrombEVAL study programme. Eur. J. Prev. Cardiol. 2015, 22, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Buller, H.R.; Prins, M.H.; Lensin, A.W.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; Agnelli, G.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkens, P.M.G.; ten Cate, H.; Büller, H.R.; Prins, M.H. Benchmark for Time in Therapeutic Range in Venous Thromboembolism: A Systemic Review and Meta-Analysis. PLoS ONE 2012, 7, e42269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beinema, M.; Brouwers, J.R.; Schalekamp, T.; Wilffert, B. Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. Thromb. Haemost. 2008, 100, 1052–1057. [Google Scholar]
- Wieloch, M.; Sjalander, A.; Frykman, V.; Rosenqvist, M.; Eriksson, N.; Svensson, P.J. Anticoagulation control in Sweden: Reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur. Heart J. 2011, 32, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; Samsa, G.P.; Cohen, S.J.; Oddone, E.Z.; Jurgelski, A.E. Improving the quality of anticoagulation of patients with atrial fibrillation in managed care organizations: Results of the managing anticoagulation services trial. Am. J. Med. 2002, 113, 42–51. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I.; Investigators, A.-E. Apixaban for extended treatment of venous thromboembolism. N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Singer, A.; McAlister, F.A.; Peeler, W.; Heran, B.S.; Drummond, N.; Manca, D.P.; Allan, G.M.; Korownyk, C.; Kolber, M.R.; et al. Quality of warfarin management in primary care: Determining the stability of international normalized ratios using a nationally representative prospective cohort. Can. Fam. Physician 2019, 65, 416–425. [Google Scholar]
- Macedo, A.F.; Bell, J.; McCarron, C.; Conroy, R.; Richardson, J.; Scowcroft, A.; Sunderland, T.; Rotheram, N. Determinants of oral anticoagulation control in new warfarin patients: Analysis using data from Clinical Practice Research Datalink. Thromb. Res. 2015, 136, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Yusuf, S.; Ezekowitz, M.D.; Alings, M.; Flather, M.; Franzosi, M.G.; Pais, P.; Dans, A.; Eikelboom, J.; Oldgren, J.; et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: An analysis of the RE-LY trial. Lancet 2010, 376, 975–983. [Google Scholar] [CrossRef]
- Wallentin, L.; Lopes, R.D.; Hanna, M.; Thomas, L.; Hellkamp, A.; Nepal, S.; Hylek, E.M.; Al-Khatib, S.M.; Alexander, J.H.; Alings, M.; et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 2013, 127, 2166–2176. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, A.M.; de Vries, F.; Plumb, J.M.; Hass, B.; Clemens, A.; van Staa, T.P. Quality of INR control and outcomes following venous thromboembolism. Clin. Appl. Thromb. Hemost. 2012, 18, 370–378. [Google Scholar] [CrossRef] [PubMed]
- van den Dries, C.J.; van Doorn, S.; Rutten, F.H.; Oudega, R.; van de Leur, S.; Elvan, A.; Oude Grave, L.; Bilo, H.J.G.; Moons, K.G.M.; Hoes, A.W.; et al. Integrated management of atrial fibrillation in primary care: Results of the ALL-IN cluster randomized trial. Eur. Heart J. 2020, 41, 2836–2844. [Google Scholar] [CrossRef]
- Prochaska, J.H.; Gobel, S.; Keller, K.; Coldewey, M.; Ullmann, A.; Lamparter, H.; Schulz, A.; Schinzel, H.; Bickel, C.; Lauterbach, M.; et al. e-Health-based management of patients receiving oral anticoagulation therapy: Results from the observational thrombEVAL study. J. Thromb. Haemost. 2017, 15, 1375–1385. [Google Scholar] [CrossRef]
- Witt, D.M.; Clark, N.P.; Kaatz, S.; Schnurr, T.; Ansell, J.E. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 187–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, G.; van Rein, N.; Bogaard, H.J.; Ende-Verhaar, Y.M.; Huisman, M.V.; Kroft, L.J.M.; van der Meer, F.J.M.; Meijboom, L.J.; Symersky, P.; Vonk Noordegraaf, A.; et al. Quality of initial anticoagulant treatment and risk of CTEPH after acute pulmonary embolism. PLoS ONE 2020, 15, e0232354. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.B.; Skjoth, F.; Sogaard, M.; Kjaeldgaard, J.N.; Lip, G.Y.; Larsen, T.B. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2017, 356, j510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohnloser, S.H.; Basic, E.; Hohmann, C.; Nabauer, M. Effectiveness and Safety of Non-Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon: Data from 61,000 Patients with Atrial Fibrillation. Thromb. Haemost. 2018, 118, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Sindet-Pedersen, C.; Langtved Pallisgaard, J.; Staerk, L.; Gerds, T.A.; Fosbol, E.L.; Torp-Pedersen, C.; Gislason, G.; Olesen, J.B. Comparative safety and effectiveness of rivaroxaban versus VKAs in patients with venous thromboembolism. A Danish nationwide registry-based study. Thromb. Haemost. 2017, 117, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.D.; Kline-Rogers, E.; Graves, C.; Puroll, E.; Gu, X.; Townsend, K.; McMahon, E.; Craig, T.; Froehlich, J.B. Structure and function of anticoagulation clinics in the United States: An AC forum membership survey. J. Thromb. Thrombolysis 2018, 46, 7–11. [Google Scholar] [CrossRef]
- Testa, S.; Paoletti, O.; Zimmermann, A.; Bassi, L.; Zambelli, S.; Cancellieri, E. The role of anticoagulation clinics in the era of new oral anticoagulants. Thrombosis 2012, 2012, 835356. [Google Scholar] [CrossRef]




| Variable | Coagulation Service (n = 254) | Regular Medical Care (n = 360) | p-Value |
|---|---|---|---|
| Age (years; mean ± SD) | 63.4 (±18.0) | 68.3 (±14.5) | 0.00035 |
| Sex (Men) | 44.9% (114/254) | 53.9% (194/360) | 0.033 |
| CHA2DS2Vasc | 3.73 (±2.02) | 4.39 (±1.98) | 0.00012 |
| HAS-BLED | 2.17 (±1.40) | 2.69 (±1.36) | <0.0001 |
| Charlson comorbidity index (mean ± SD) | 4.42 (±2.71) | 5.67 (±2.56) | <0.0001 |
| Sociodemographic Factors | |||
| Partnership | 63.8% (162/254) | 64.2% (231/360) | 0.93 |
| >2 Persons living in household | 16.5% (42/254) | 13.6% (49/360) | 0.36 |
| Children (at least one) | 73.6% (187/254) | 82.2% (296/360) | 0.012 |
| Nursing home inhabitants | 6.7% (17/254) | 4.7% (17/360) | 0.37 |
| Immigrants | 5.1% (13/254) | 10.8% (39/360) | 0.012 |
| Working | 30.4% (77/253) | 16.4% (59/360) | <0.0001 |
| Higher School Certificate (Abitur) | 18.1% (46/254) | 15.3% (55/360) | 0.38 |
| School education (<10 years) | 63.0% (160/254) | 68.6% (247/360) | 0.17 |
| Classical Cardiovascular Risk Factors | |||
| Arterial hypertension | 59.8% (152/254) | 71.9% (259/360) | 0.0023 |
| Diabetes | 17.4% (44/253) | 26.7% (96/360) | 0.0082 |
| Dyslipidemia | 30.3% (77/254) | 51.4% (185/360) | <0.0001 |
| Family history of myocardial infarction or stroke | 29.5% (75/254) | 39.7% (143/360) | <0.0001 |
| Obesity (BMI ≥ 30 kg/m2) | 32.3% (82/254) | 34.2% (123/360) | 0.66 |
| Smoking (currently) | 7.1% (18/254) | 10.6% (38/360) | 0.16 |
| Concomitant Diseases | |||
| Atrial fibrillation | 22.4% (57/254) | 46.5% (166/357) | <0.0001 |
| Cancer | 22.9% (56/245) | 20.1% (71/354) | 0.42 |
| Chronic lung disease | 14.6% (37/254) | 28.8% (102/354) | <0.0001 |
| Coronary artery disease | 16.7% (42/252) | 37.5% (127/339) | <0.0001 |
| Heart failure | 13.8% (35/254) | 36.1% (127/352) | <0.0001 |
| History of myocardial infarction | 9.1% (23/253) | 21.8% (78/358) | <0.0001 |
| History of stroke or transient ischemic attack | 12.6% (32/254) | 14.2% (51/359) | 0.63 |
| Liver disease | 3.5% (9/254) | 5.1% (18/356) | <0.0001 |
| Peripheral artery disease | 6.3% (16/254) | 22.9% (79/345) | <0.0001 |
| Renal disease | 14.2% (36/254) | 23.7% (85/358) | 0.0038 |
| Sleep apnea | 4.8% (12/250) | 9.8% (34/346) | 0.029 |
| Thrombophilia | 12.6% (32/254) | 9.2% (33/360) | 0.18 |
| Quality of OAC therapy | |||
| TTR (median IQR) | 76.9% (63.2–87.1%) | 69.5% (52.3–85.6%) | <0.001 |
| TuTR (median IQR) | 6.4% (0.8–15.0%) | 13.3% (2.2–27.9%) | <0.001 |
| ToTR(median IQR) | 10.6% (3.9–21.1%) | 7.3% (0–21.7%) | 0.033 |
| Stable anticoagulation control | 86.2% (207/240) | 75.4(193/256) | 0.0030 |
| Concomitant medication | |||
| Anti-platelet agents | 19.7% (50/254) | 18.1% (65/360) | 0.67 |
| Non-steroidal anti-inflammatory drugs | 11.8% (30/254) | 5.8% (21/360) | 0.011 |
| Proton pump inhibitor | 38.6% (98/254) | 35.0% (126/360) | 0.39 |
| Statin | 24.4% (62/254) | 32.5% (117/360) | 0.031 |
| Events in RMC | Rate RMC | Events in CS | Rate CS | Rate Ratio (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Primary outcome | 150 | 25.16 | 10 | 3.70 | 6.80 (3.60–14.47) | <0.001 |
| All-cause mortality | 65 | 10.90 | 5 | 1.85 | 5.89 (2.40–18.75) | <0.001 |
| Clinically relevant bleeding | 60 | 10.06 | 4 | 1.48 | 6.80 (2.52–25.76) | <0.001 |
| Thromboembolic events | 25 | 4.19 | 1 | 0.37 | 11.33 (1.85–465.26) | 0.0015 |
| Hospitalization | 348 | 58.36 | 62 | 22.94 | 2.54 (1.94–3.39) | <0.001 |
| Variables of Adjustment | Adjustment for Age and Sex | Adjustment for Age, Sex and Charlson Index | Adjustment for Age, Sex Charlson Index and TTR | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Primary outcome | 5.39 (2.81–10.33) | <0.0001 | 5.04 (2.62–9.69) | <0.0001 | 5.01 (2.56–9.80) | <0.0001 |
| All-cause mortality | 5.54 (2.22–13.84) | <0.001 | 4.85 (1.94–12.15) | <0.001 | 4.77 (1.86–12.27) | 0.0012 |
| Clinically relevant bleeding | 5.31 (1.89–14.89) | 0.0015 | 5.38 (1.91–15.14) | 0.0014 | 6.29 (2.20–17.96) | <0.001 |
| Thromboembolic events | 6.41 (1.51–27.24) | 0.012 | 6.83 (1.60–29.12) | 0.0094 | 6.31 (1.43–27.76) | 0.015 |
| Hospitalization | 1.84 (1.34–2.55) | <0.001 | 1.76 (1.27–2.44) | <0.001 | 1.90 (1.35–2.68) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, K.; Göbel, S.; ten Cate, V.; Panova-Noeva, M.; Eggebrecht, L.; Nagler, M.; Coldewey, M.; Foebel, M.; Bickel, C.; Lauterbach, M.; et al. Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study. J. Clin. Med. 2020, 9, 3281. https://doi.org/10.3390/jcm9103281
Keller K, Göbel S, ten Cate V, Panova-Noeva M, Eggebrecht L, Nagler M, Coldewey M, Foebel M, Bickel C, Lauterbach M, et al. Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study. Journal of Clinical Medicine. 2020; 9(10):3281. https://doi.org/10.3390/jcm9103281
Chicago/Turabian StyleKeller, Karsten, Sebastian Göbel, Vincent ten Cate, Marina Panova-Noeva, Lisa Eggebrecht, Markus Nagler, Meike Coldewey, Maike Foebel, Christoph Bickel, Michael Lauterbach, and et al. 2020. "Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study" Journal of Clinical Medicine 9, no. 10: 3281. https://doi.org/10.3390/jcm9103281
APA StyleKeller, K., Göbel, S., ten Cate, V., Panova-Noeva, M., Eggebrecht, L., Nagler, M., Coldewey, M., Foebel, M., Bickel, C., Lauterbach, M., Espinola-Klein, C., Lackner, K. J., ten Cate, H., Münzel, T., S. Wild, P., & H. Prochaska, J. (2020). Telemedicine-Based Specialized Care Improves the Outcome of Anticoagulated Individuals with Venous Thromboembolism—Results from the thrombEVAL Study. Journal of Clinical Medicine, 9(10), 3281. https://doi.org/10.3390/jcm9103281