Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy
Abstract
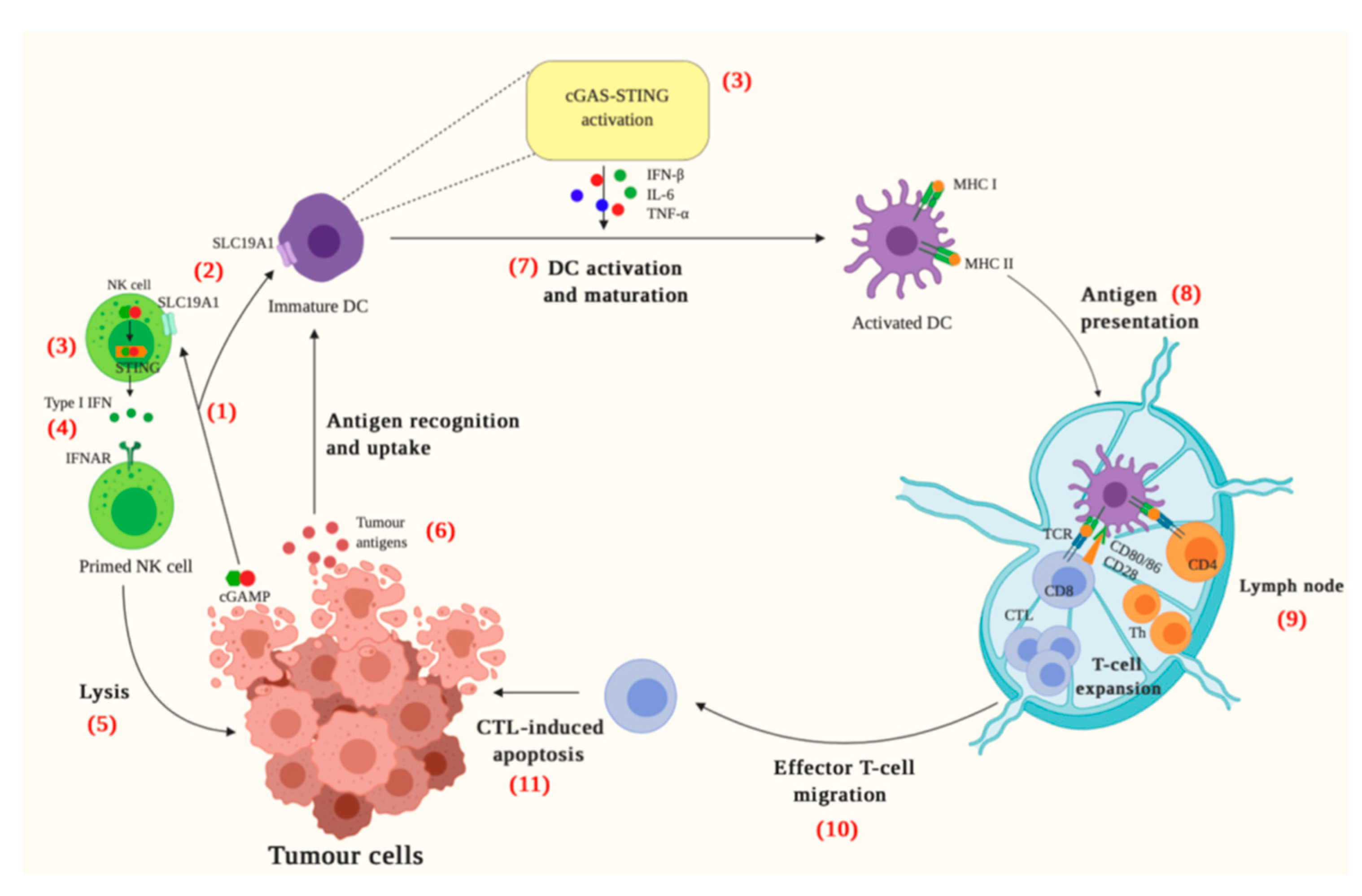
:1. Introduction
2. The cGAS-STING Pathway

3. cGAS-STING and Cancer Immunity
4. STING Activating Drugs
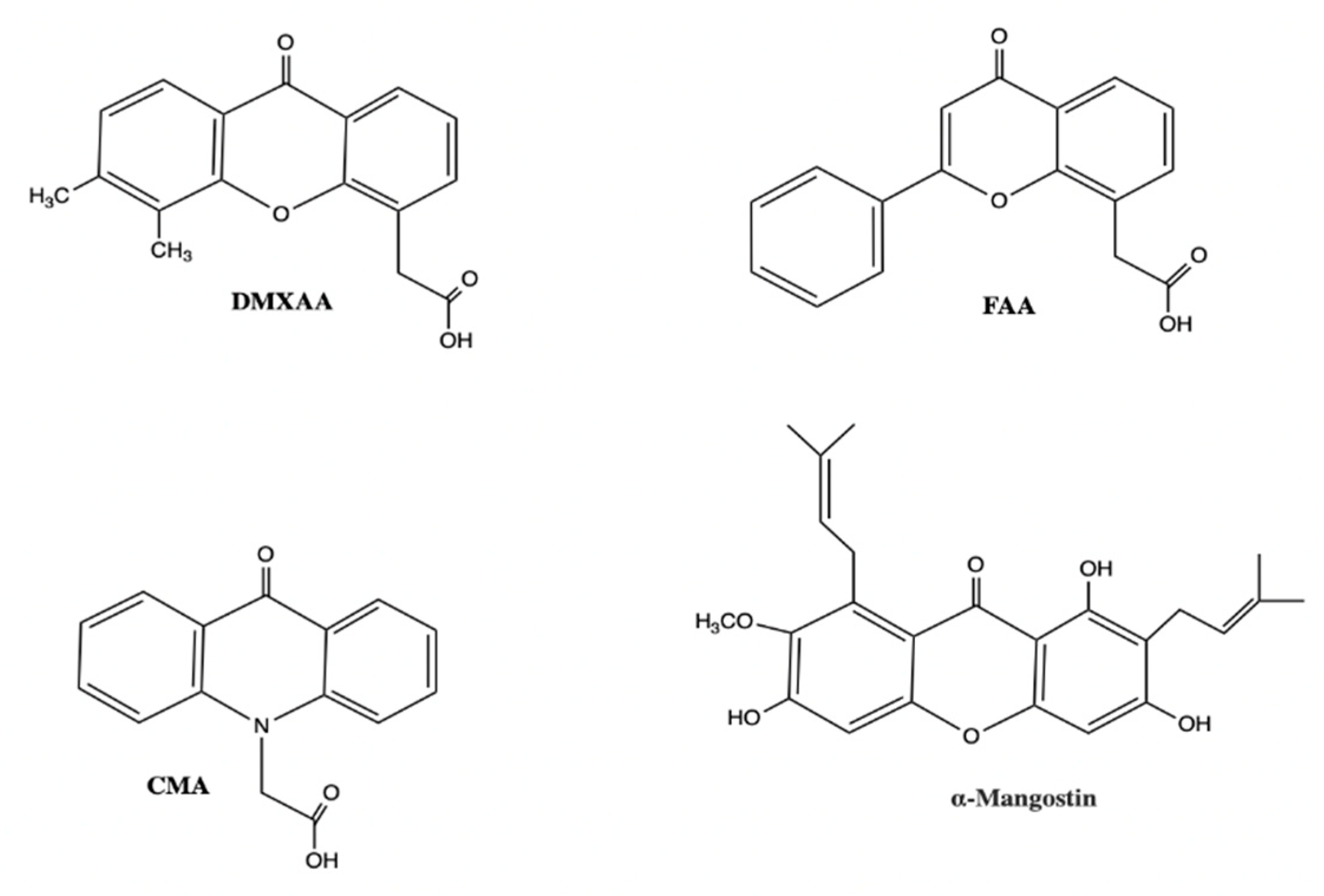
4.1. DMXAA
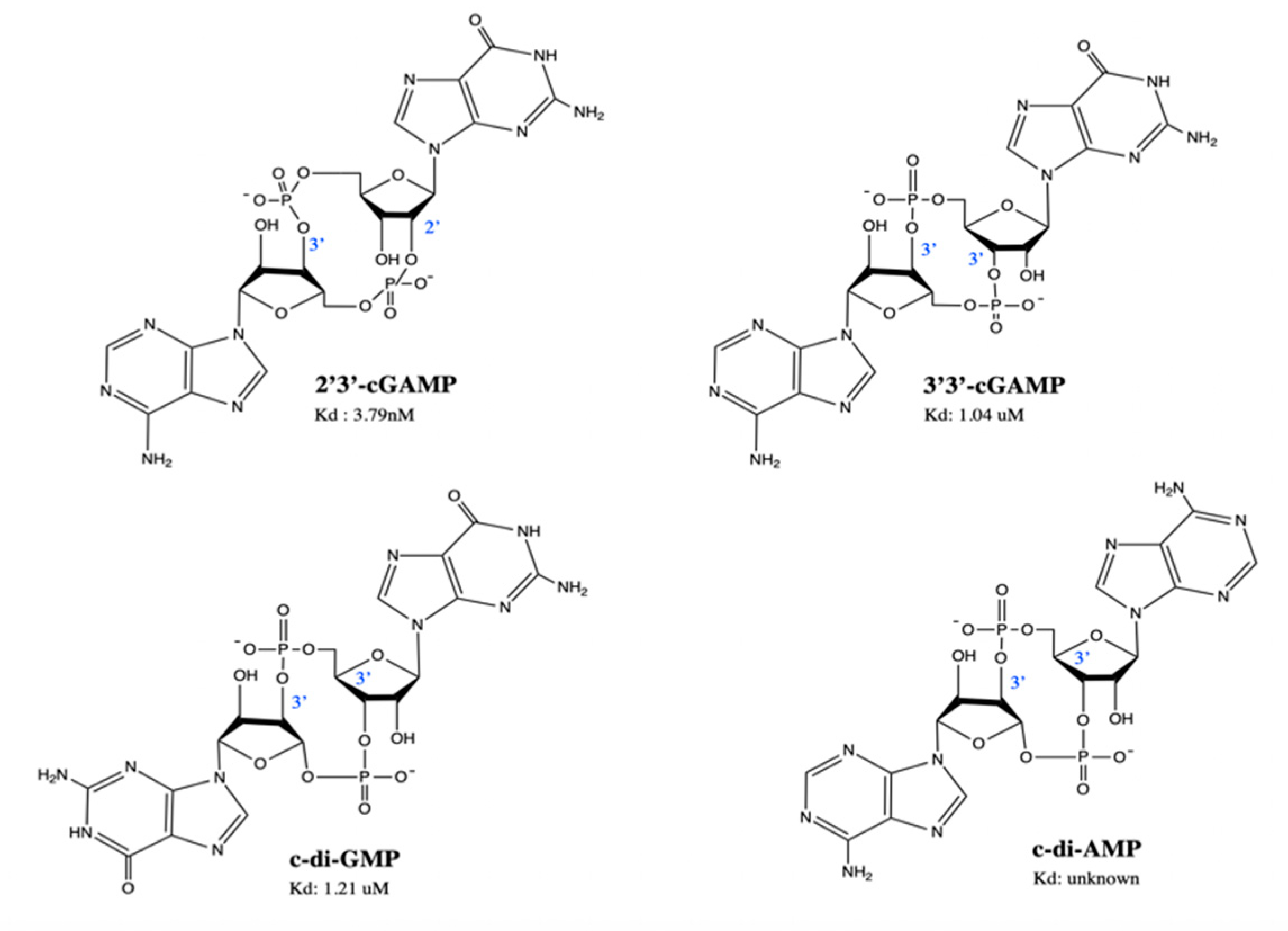
4.2. Natural STING Agonists—Natural CDNs
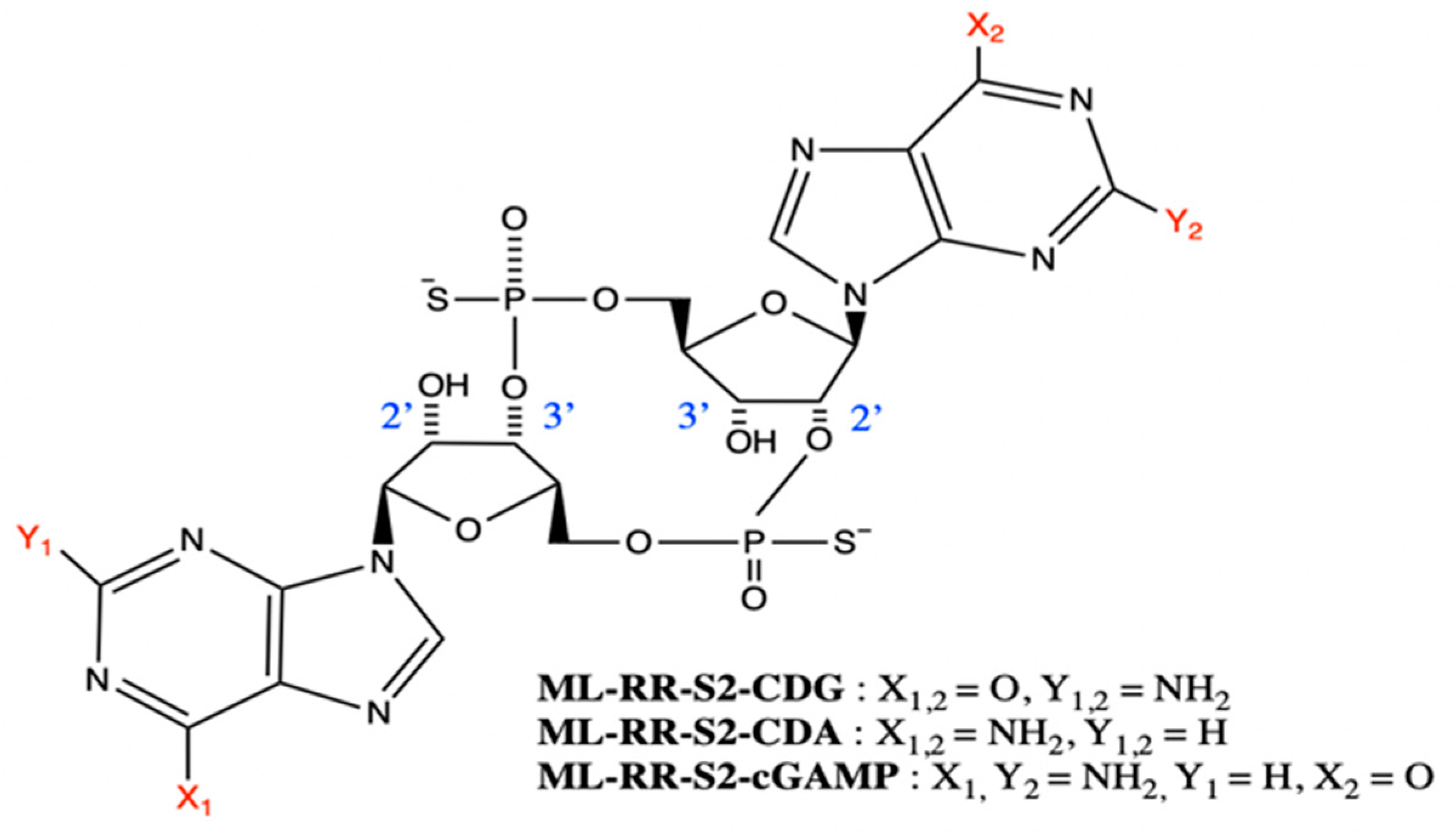
4.3. Synthetic CDNs
4.4. Additional Non-Nucleotidyl Small Molecule STING Agonists
5. Combination Therapy—STING Agonists with ICI
6. STING Agonists in Clinical Trials
6.1. ADU-S100 (ML-RR-S2-CDA)
6.2. MK-1454
6.3. MK-2118
6.4. BMS-986301
6.5. GSK-3745417
6.6. SB-11285
6.7. IMSA-101
6.8. E7766
7. Challenges to STING Activating Drug Development
8. Future Directions
8.1. Liposomes
8.2. Polymers
8.3. Hydrogels
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helmink, B.A.; Gaudreau, P.-O.; Wargo, J. Immune Checkpoint Blockade across the Cancer Care Continuum. Immunity 2018, 48, 1077–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesterhuis, W.J.; Haanen, J.B.A.G.; Punt, C.J.A. Cancer immunotherapy—Revisited. Nat. Rev. Drug Discov. 2011, 10, 591–600. [Google Scholar] [CrossRef]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasić, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.-J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti–Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef]
- Ahn, J.; Xia, T.; Capote, A.R.; Betancourt, D.M.; Barber, G.N. Extrinsic Phagocyte-Dependent STING Signaling Dictates the Immunogenicity of Dying Cells. Cancer Cell 2018, 33, 862–873.e5. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell 2017, 170, 1055–1056. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.; Song, Z.; Shen, A.; Chen, T.; Zhang, A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Q.; Kuss, P.; Maliga, Z.; Millán, J.L.; Wu, H.; Mitchison, T.J. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 2014, 10, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrales, L.; McWhirter, S.M.; Dubensky, T.W.; Gajewski, T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Investig. 2016, 126, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nat. Cell Biol. 2009, 461, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Schiavoni, G.; Mattei, F.; Gabriele, L. Type I Interferons as Stimulators of DC-Mediated Cross-Priming: Impact on Anti-Tumor Response. Front. Immunol. 2013, 4, 483. [Google Scholar] [CrossRef] [Green Version]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.-R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [Green Version]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2012, 339, 786–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] Is the Metazoan Second Messenger Produced by DNA-Activated Cyclic GMP-AMP Synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal Roles of cGAS-cGAMP Signaling in Antiviral Defense and Immune Adjuvant Effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, G.; Zhang, C.; Chen, Z.J.; Bai, X.-C.; Zhang, X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP–AMP. Nat. Cell Biol. 2019, 567, 389–393. [Google Scholar] [CrossRef]
- Zhao, B.; Du, F.; Xu, P.; Shu, C.; Sankaran, B.; Bell, S.L.; Liu, M.; Lei, Y.; Gao, X.; Fu, X.; et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nat. Cell Biol. 2019, 569, 718–722. [Google Scholar] [CrossRef]
- Gomelsky, M. cAMP, c-di-GMP, c-di-AMP and now cGMP: Bacteria use them all! Mol. Microbiol. 2011, 79, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Mukai, K.; Konno, H.; Akiba, T.; Uemura, T.; Waguri, S.; Kobayashi, T.; Barber, G.N.; Arai, H.; Taguchi, T. Activation of STING requires palmitoylation at the Golgi. Nat. Commun. 2016, 7, 11932. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.-C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nat. Cell Biol. 2019, 567, 394–398. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Wu, J.; Zhang, X.; Sun, L.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Containing Mixed Phosphodiester Linkages Is An Endogenous High-Affinity Ligand for STING. Mol. Cell 2013, 51, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Fuertes, M.B.; Woo, S.-R.; Burnett, B.; Fu, Y.-X.; Gajewski, T.F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013, 34, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103 + Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef] [Green Version]
- Klarquist, J.; Hennies, C.M.; Lehn, M.A.; Reboulet, R.A.; Feau, S.; Janssen, E.M. STING-Mediated DNA Sensing Promotes Antitumor and Autoimmune Responses to Dying Cells. J. Immunol. 2014, 193, 6124–6134. [Google Scholar] [CrossRef]
- Zhou, R.; Xie, X.; Li, X.; Qin, Z.; Wei, C.; Liu, J.; Luo, Y. The triggers of the cGAS-STING pathway and the connection with inflammatory and autoimmune diseases. Infect. Genet. Evol. 2019, 77, 104094. [Google Scholar] [CrossRef]
- Rueckert, C.; Rand, U.; Roy, U.; Kasmapour, B.; Strowig, T.; Guzman, A.C. Cyclic dinucleotides modulate induced type I IFN responses in innate immune cells by degradation of STING. FASEB J. 2017, 31, 3107–3115. [Google Scholar] [CrossRef] [Green Version]
- Gonugunta, V.K.; Sakai, T.; Pokatayev, V.; Yang, K.; Wu, J.; Dobbs, N.; Yan, N. Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-tumor Response. Cell Rep. 2017, 21, 3234–3242. [Google Scholar] [CrossRef] [Green Version]
- Sundararaman, S.K.; Barbie, D.A. Tumor cGAMP Awakens the Natural Killers. Immunity 2018, 49, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS–STING Pathway in Cancer. Cancer Discov. 2019, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z.; et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nat. Cell Biol. 2017, 550, 402–406. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301–e368. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nat. Cell Biol. 2012, 482, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nat. Cell Biol. 2017, 548, 466–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, K.J.; Carroll, P.; Martin, C.-A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nat. Cell Biol. 2017, 548, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, S.; Ivanova, E.; Guo, S.; Yoshida, R.; Campisi, M.; Sundararaman, S.K.; Tange, S.; Mitsuishi, Y.; Thai, T.C.; Masuda, S.; et al. Suppression of STING Associated with LKB1 Loss in KRAS-Driven Lung Cancer. Cancer Discov. 2018, 9, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, C.; Cordova, A.F.; Hess, G.T.; Bassik, M.C.; Li, L. SLC19A1 Is an Importer of the Immunotransmitter cGAMP. Mol. Cell 2019, 75, 372–381.e5. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.; Mao, A.J.; Lensink-Vasan, M.; Wang, L.; Vance, E.R.; Raulet, D.H. Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response. Immun. 2018, 49, 754–763.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carozza, J.A.; Böhnert, V.; Shaw, K.E.; Nguyen, K.C.; Skariah, G.; Brown, J.A.; Rafat, M.; von Eyben, R.; Graves, E.E.; Glenn, J.S.; et al. Extracellular 2’3’-cGAMP is an immunotransmitter produced by cancer cells and regulated by ENPP1. bioRxiv 2019. bioRxiv:539312. [Google Scholar]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Man, S.M.; Gurung, P.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.-D. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 2014, 193, 4779–4782. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.K.; Skouboe, M.K.; Boularan, C.; Vernejoul, F.; Lioux, T.; Leknes, S.L.; Berthelsen, M.F.; Riedel, M.; Cai, H.; Joseph, J.V.; et al. The cGAS-STING pathway is a therapeutic target in a preclinical model of hepatocellular carcinoma. Oncogene 2019, 39, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. Identifying and Overcoming Immune Resistance Mechanisms in the Melanoma Tumor Microenvironment. Clin. Cancer Res. 2006, 12, 2326s–2330s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagès, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients with Early-Stage Colorectal Cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.-C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.-H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2009, 29, 1093–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, S.M.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-Infiltrating CD8+ Lymphocytes Predict Clinical Outcome in Breast Cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; De Vroom, E.; Van Der Marel, G.A.; Van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nat. Cell Biol. 1987, 325, 279–281. [Google Scholar] [CrossRef]
- Ahn, J.; Xia, T.; Konno, H.; Konno, K.; Ruiz, P.; Barber, G.N. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 2014, 5, 5166. [Google Scholar] [CrossRef] [Green Version]
- Baird, J.R.; Dietsch, G.N.; Florio, V.; Gallatin, M.; Knox, C.D.; Odingo, J.; Crittenden, M.; Gough, M.J. "MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo". Society for Immunotherapy of Cancer 2018 Annual Meeting Posters. 7. Available online: https://digitalcommons.psjhealth.org/sitc2018/7 (accessed on 10 October 2020).
- Gao, P.; Ascano, M.; Zillinger, T.; Wang, W.; Dai, P.; Serganov, A.A.; Gaffney, B.L.; Shuman, S.; Jones, R.A.; Deng, L.; et al. Structure-Function Analysis of STING Activation by c[G(2′,5′)pA(3′,5′)p] and Targeting by Antiviral DMXAA. Cell 2013, 154, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Conlon, J.; Burdette, D.L.; Sharma, S.; Bhat, N.; Thompson, M.; Jiang, Z.; Rathinam, V.A.K.; Monks, B.; Jin, T.; Xiao, T.S.; et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 2013, 190, 5216–5225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Li, L.; Maliga, Z.; Yin, Q.; Wu, H.; Mitchison, T.J. Anticancer Flavonoids Are Mouse-Selective STING Agonists. ACS Chem. Biol. 2013, 8, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prantner, D.; Perkins, D.J.; Lai, W.; Williams, M.S.; Sharma, S.; Fitzgerald, K.A.; Vogel, S.N. 5,6-Dimethylxanthenone-4-acetic Acid (DMXAA) Activates Stimulator of Interferon Gene (STING)-dependent Innate Immune Pathways and Is Regulated by Mitochondrial Membrane Potential. J. Biol. Chem. 2012, 287, 39776–39788. [Google Scholar] [CrossRef] [Green Version]
- Baguley, B.C.; Ching, L.-M. DMXAA: An antivascular agent with multiple host responses. Int. J. Radiat. Oncol. 2002, 54, 1503–1511. [Google Scholar] [CrossRef]
- Baguley, B.C. Antivascular therapy of cancer: DMXAA. Lancet Oncol. 2003, 4, 141–148. [Google Scholar] [CrossRef]
- Lara, P.N.; Douillard, J.-Y.; Nakagawa, K.; Von Pawel, J.; McKeage, M.J.; Albert, I.; Losonczy, G.; Reck, M.; Heo, D.-S.; Fan, X.; et al. Randomized Phase III Placebo-Controlled Trial of Carboplatin and Paclitaxel With or Without the Vascular Disrupting Agent Vadimezan (ASA404) in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 2965–2971. [Google Scholar] [CrossRef]
- Früh, M.; Cathomas, R.; Siano, M.; Tscherry, G.; Zippelius, A.; Mamot, C.; Erdmann, A.; Krasniqi, F.; Rauch, D.; Simcock, M.; et al. Carboplatin and Paclitaxel Plus ASA404 as First-Line Chemotherapy for Extensive-Stage Small-Cell Lung Cancer: A Multicenter Single Arm Phase II Trial (SAKK 15/08). Clin. Lung Cancer 2013, 14, 34–39. [Google Scholar] [CrossRef]
- Shih, A.Y.; Damm-Ganamet, K.L.; Mirzadegan, T. Dynamic Structural Differences between Human and Mouse STING Lead to Differing Sensitivity to DMXAA. Biophys. J. 2018, 114, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Karaolis, D.K.R.; Cheng, K.; Lipsky, M.; Elnabawi, A.; Catalano, J.; Hyodo, M.; Hayakawa, Y.; Raufman, J.-P. 3′,5′-Cyclic diguanylic acid (c-di-GMP) inhibits basal and growth factor-stimulated human colon cancer cell proliferation. Biochem. Biophys. Res. Commun. 2005, 329, 40–45. [Google Scholar] [CrossRef]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. c-di-AMP Secreted by Intracellular Listeria monocytogenes Activates a Host Type I Interferon Response. Science 2010, 328, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhirter, S.M.; Barbalat, R.; Monroe, K.M.; Fontana, M.F.; Hyodo, M.; Joncker, N.T.; Ishii, K.J.; Akira, S.; Colonna, M.; Chen, Z.J.; et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 2009, 206, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nat. Cell Biol. 2011, 478, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; KuoLee, R.; Yan, H. The potential of 3′,5′-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine 2010, 28, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Kanne, D.B.; Leong, M.; Glickman, L.H.; McWhirter, S.M.; Lemmens, E.; Mechette, K.; Leong, J.J.; Lauer, P.; Liu, W.; et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015, 7, 283ra52. [Google Scholar] [CrossRef] [Green Version]
- Berger, G.; Marloye, M.; Lawler, S.E. Pharmacological Modulation of the STING Pathway for Cancer Immunotherapy. Trends Mol. Med. 2019, 25, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.-H.A.; Zundell, J.A.; Ranatunga, S.; Lin, C.; Nefedova, Y.; Del Valle, J.R.; Hu, C.-C.A. Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res. 2016, 76, 2137–2152. [Google Scholar] [CrossRef] [Green Version]
- Ohkuri, T.; Kosaka, A.; Ishibashi, K.; Kumai, T.; Hirata, Y.; Ohara, K.; Nagato, T.; Oikawa, K.; Aoki, N.; Harabuchi, Y.; et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol. Immunother. 2017, 66, 705–716. [Google Scholar] [CrossRef]
- Su, T.; Zhang, Y.; Valerie, K.; Wang, X.-Y.; Lin, S.; Zhu, G. STING activation in cancer immunotherapy. Theranostics 2019, 9, 7759–7771. [Google Scholar] [CrossRef]
- Endo, A.; Kim, D.-S.; Huang, K.-C.; Hao, M.-H.; Mathieu, S.; Choi, H.-W.; Majumder, U.; Zhu, X.; Shen, Y.; Sanders, K.; et al. Abstract 4456: Discovery of E7766: A representative of a novel class of macrocycle-bridged STING agonists (MBSAs) with superior potency and pan-genotypic activity. Cancer Res. 2019, 79 (Suppl. 13), 4456. [Google Scholar]
- Huang, K.-C.; Endo, A.; McGrath, S.; Chandra, D.; Wu, J.; Kim, D.-S.; Albu, D.; Ingersoll, C.; Tendyke, K.; Loiacono, K.; et al. Abstract 3269: Discovery and characterization of E7766, a novel macrocycle-bridged STING agonist with pan-genotypic and potent antitumor activity through intravesical and intratumoral administration. Cancer Res. 2019, 79 (Suppl. 13), 3269. [Google Scholar]
- Futami, H.; Eader, A.L.; Komschlies, K.L.; Bull, R.; Gruys, E.M.; Ortaldo, J.R.; Young, H.A.; Wiltrout, R.H. Flavone acetic acid directly induces expression of cytokine genes in mouse splenic leukocytes but not in human peripheral blood leukocytes. Cancer Res. 1991, 51, 51. [Google Scholar]
- Cavlar, T.; Deimling, T.; Ablasser, A.; Hopfner, K.-P.; Hornung, V. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J. 2013, 32, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Pei, J.; Luo, Q.; Zeng, X.; Li, Q.; Yang, Z.; Quan, J. Identification of α-Mangostin as an Agonist of Human STING. ChemMedChem 2018, 13, 2057–2064. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Tang, L.; Su, Q.; Hwang, N.; Sehgal, M.; Cheng, J.; Ma, J.; Zhang, X.; Tan, Y.; et al. Discovery and Mechanistic Study of a Novel Human-Stimulator-of-Interferon-Genes Agonist. ACS Infect. Dis. 2019, 5, 1139–1149. [Google Scholar] [CrossRef]
- Liu, B.; Tang, L.; Zhang, X.; Ma, J.; Sehgal, M.; Cheng, J.; Zhang, X.; Zhou, Y.; Du, Y.; Kulp, J.; et al. A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antivir. Res. 2017, 147, 37–46. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Lemos, H.; Chandler, P.R.; Pacholczyk, G.; Baban, B.; Barber, G.N.; Hayakawa, Y.; McGaha, T.L.; Ravishankar, B.; et al. Cutting Edge: DNA Sensing via the STING Adaptor in Myeloid Dendritic Cells Induces Potent Tolerogenic Responses. J. Immunol. 2013, 191, 3509–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, M.; Middya, S.; Basu, S.; Ghosh, R.; Pryde, D.; Yadav, D.; Shrivastava, R.; Surya, A. Small Molecule Modulators of Human Sting. Curadev Pharma Ltd. WO2018234805A1, 27 December 2018. [Google Scholar]
- Banerjee, M.; Middya, S.; Basu, S.; Ghosh, R.; Pryde, D.; Yadav, D.; Shrivastava, R.; Surya, A. Small molecule modulators of human sting. Curadev Pharma Ltd. WO2018234808A1, 27 December 2018. [Google Scholar]
- Banerjee, M.; Middya, S.; Basu, S.; Ghosh, R.; Pryde, D.; Yadav, D.; Shrivastava, R.; Surya, A. Small molecule modulators of human sting. Curadev Pharma Ltd. WO2018234807A1, 27 December 2018. [Google Scholar]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.-Y.; Tran, J.-L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nat. Cell Biol. 2018, 564, 439–443. [Google Scholar] [CrossRef]
- Cemerski, S.; Cumming, J.N.; Kopinja, J.E.; Perera, S.A.; Trotter, B.W.; Tse, A.N.C. Benzo[b]thiophene STING agonists for cancer treatment. Merck Sharp & Dohme Corp, 2019. WO2019027858A1, 7 February 2019. [Google Scholar]
- Pan, B.S.; Perera, S.A.; Piesvaux, J.A.; Presland, J.P.; Schroeder, G.K.; Cumming, J.N.; Trotter, B.W.; Altman, M.D.; Buevich, A.V.; Cash, B.; et al. An orally available non-nucleotide STING agonist with antitumor activity. Science 2020, 369, 6506. [Google Scholar]
- Chin, E.N.; Yu, C.; Vartabedian, V.F.; Jia, Y.; Kumar, M.; Gamo, A.M.; Vernier, W.; Ali, S.H.; Kissai, M.; Lazar, D.C.; et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science 2020, 369, 993–999. [Google Scholar]
- Cemerski, S.; Cumming, J.N.; Kopinja, J.E.; Perera, S.A.; Trotter, B.W.; Tse, A.N.C. Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment. Merck Sharp & Dohme Corp, 2019. WO2019027857A1, 7 February 2019. [Google Scholar]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Xiao-Feng, H.; Guan-Jun, D.; Er-Ling, H.; Sheng, C.; Ting-Ting, W.; Qin-Gang, H.; Ni, Y.; Hou, Y. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 2494–2503. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.W.; Barbie, A.D.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, A.; Robert, C.; Hodi, F.S.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.-J.; Weber, J.S.; Zarour, H.M.; Kefford, R.; Loboda, A.; et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J. Clin. Oncol. 2015, 33, 3001. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Weiss, J.; Eder, J.P.; Yearley, J.; Murphy, E.; Nebozhyn, M.; McClanahan, T.; Ayers, M.; Lunceford, J.K.; et al. Inflamed-phenotype gene expression signatures to predict benefit from the anti-PD-1 antibody pembrolizumab in PD-L1+ head and neck cancer patients. J. Clin. Oncol. 2015, 33, 6017. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Longhi, M.P.; Trumpfheller, C.; Idoyaga, J.; Caskey, M.; Matos, I.; Kluger, C.; Salazar, A.M.; Colonna, M.; Steinman, R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009, 206, 1589–1602. [Google Scholar] [CrossRef]
- Foote, J.B.; Kok, M.; Leatherman, J.M.; Armstrong, T.D.; Marcinkowski, B.; Ojalvo, L.S.; Kanne, D.B.; Jaffee, E.M.; Dubensky, T.W.; Emens, L.A. A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice. Cancer Immunol. Res. 2017, 5, 468–479. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.; Clavijo, P.E.; Davis, R.; Cash, H.; Van Waes, C.; Kim, Y.; Allen, C.T. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol. Res. 2016, 4, 1061–1071. [Google Scholar] [CrossRef] [Green Version]
- Ager, C.R.; Reilley, M.J.; Nicholas, C.; Bartkowiak, T.; Jaiswal, A.R.; Curran, M.A. Intratumoral STING Activation with T-cell Checkpoint Modulation Generates Systemic Antitumor Immunity. Cancer Immunol. Res. 2017, 5, 676–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meric-Bernstam, F.; Sandhu, S.K.; Hamid, O.; Spreafico, A.; Kasper, S.; Dummer, R.; Shimizu, T.; Steeghs, N.; Lewis, N.; Talluto, C.C.; et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J. Clin. Oncol. 2019, 37, 2507. [Google Scholar] [CrossRef]
- Adams, B. Aduro Stung as Novartis Drops Work on STING Drug. Available online: https://www.fiercebiotech.com/biotech/aduro-stung-as-novartis-drops-work-sting-drug (accessed on 10 October 2020).
- Harrington, K.; Brody, J.; Ingham, M.; Strauss, J.; Cemerski, S.; Wang, M.; Tse, A.; Khilnani, A.; Marabelle, A.; Golan, T. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann. Oncol. 2018, 29, viii712. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. Bristol-Myers Squibb to Acquire IFM Therapeutics to Strengthen Oncology Pipeline Focus on Innate Immunity. Available online: https://news.bms.com/news/details/2017/Bristol-Myers-Squibb-to-Acquire-IFM-Therapeutics-to-Strengthen-Oncology-Pipeline-Focus-on-Innate-Immunity/default.aspx (accessed on 10 October 2020).
- Schieven, G.; Brown, J.; Swanson, J.; Stromko, C.; Ching-Ping, H.; Zhang, R.; Li-Wang, B.; Qiu, H.; Sun, H.; Fink, B.; et al. Preclinical characterization of BMS-986301, a differentiated STING agonist with robust antitumor activity as monotherapy or in combination with anti–PD-1. In Proceedings of the 33rd Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2018), Washington, DC, USA, 7–11 November 2018. [Google Scholar]
- Challa, S.; Sheri, A.; Weitzel, D.; Cleary, D.; Wood, E.; Thorpe, L.; Zhou, S.; Afdhal, N.; Iyer, R. “Pharmacodynamic and preclinical studies of SB 11285, a highly potent, and systemically bioavailable STING agonist as a novel immuno-therapeutic agent”. AACR Tumor Immunology and Immunotherapy, 2017. Posters. A25. Available online: https://springbankpharm.com/wp-content/uploads/2013/06/Challa_AACR-Poster_09262017.pdf (accessed on 10 October 2020).
- Spring Bank Pharmaceuticals. Spring Bank Announces Clinical Collaboration to Evaluate SB 11285 in Combination with PD-L1 Checkpoint Inhibitor in Patients with Advanced Solid Tumors. Available online: https://www.globenewswire.com/news-release/2020/02/25/1990024/0/en/Spring-Bank-Announces-Clinical-Collaboration-to-Evaluate-SB-11285-in-Combination-with-PD-L1-Checkpoint-Inhibitor-in-Patients-with-Advanced-Solid-Tumors.html (accessed on 10 October 2020).
- Lemos, H.; Ou, R.; McCardle, C.; Lin, Y.; Calver, J.; Minett, J.; Chadli, A.; Huang, L.; Mellor, A.L. Overcoming resistance to STING agonist therapy to incite durable protective antitumor immunity. J. Immunother. Cancer 2020, 8, e001182. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, B.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Vannier, E.; Poltorak, A. Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J. Immunol. 2017, 199, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, G.; Brendel, V.P.; Shu, C.; Li, P.; Palanathan, S.; Kao, C.C. Single Nucleotide Polymorphisms of Human STING Can Affect Innate Immune Response to Cyclic Dinucleotides. PLoS ONE 2013, 8, e77846. [Google Scholar] [CrossRef]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.S.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nat. Cell Biol. 2016, 533, 493–498. [Google Scholar] [CrossRef]
- Sivick, K.E.; Desbien, A.L.; Glickman, L.H.; Reiner, G.L.; Corrales, L.; Surh, N.H.; Hudson, T.E.; Vu, T.U.; Francica, B.J.; Banda, T.; et al. Magnitude of Therapeutic STING Activation Determines CD8+ T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2018, 25, 3074–3085.e5. [Google Scholar] [CrossRef]
- Smith, T.T.; Moffett, H.F.; Stephan, S.B.; Opel, C.F.; Dumigan, A.G.; Jiang, X.; Pillarisetty, V.G.; Pillai, S.P.S.; Wittrup, K.D.; Stephan, M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Investig. 2017, 127, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.R.; Sen, R.; Sunshine, J.C.; Pardoll, D.M.; Green, J.J.; Kim, Y.J. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Watkins-Schulz, R.; Junkins, R.D.; David, C.N.; Johnson, B.M.; Montgomery, S.A.; Peine, K.J.; Darr, D.B.; Yuan, H.; McKinnon, K.P.; et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1–insensitive models of triple-negative breast cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano 2014, 9, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, X.; Liang, X.; Yang, J.; Zhang, C.; Kong, D.; Wang, W. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019, 85, 1–26. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyabe, H.; Hyodo, M.; Sato, Y.; Hayakawa, Y.; Harashima, H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release 2015, 216, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Miyabe, H.; Hyodo, M.; Nakamura, T.; Sato, Y.; Hayakawa, Y.; Harashima, H. A new adjuvant delivery system ‘cyclic di-GMP/YSK05 liposome’ for cancer immunotherapy. J. Control. Release 2014, 184, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1. [Google Scholar] [CrossRef]
- Wang-Bishop, L.; Wehbe, M.; Shae, D.; James, J.; Hacker, B.C.; Garland, K.; Chistov, P.P.; Rafat, M.; Balko, J.M.; Wilson, J.T. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J. Immunother. Cancer 2020, 8, e000282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, M.A.; Junkins, R.D.; Gallovic, M.D.; Johnson, B.M.; Johnson, M.M.; Macintyre, A.N.; Sempowski, G.D.; Bachelder, E.M.; Ting, J.P.-Y.; Ainslie, K.M. Acetalated Dextran Microparticles for Codelivery of STING and TLR7/8 Agonists. Mol. Pharm. 2018, 15, 4933–4946. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jang, H.-E.; Kang, Y.Y.; Kim, J.; Ahn, J.-H.; Mok, H. Submicron-sized hydrogels incorporating cyclic dinucleotides for selective delivery and elevated cytokine release in macrophages. Acta Biomater. 2016, 29, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef] [Green Version]
- Baird, J.R.; Bell, R.B.; Troesch, V.; Friedman, D.J.; Bambina, S.; Kramer, G.; Blair, T.C.; Medler, T.R.; Wu, Y.; Sun, Z.; et al. Evaluation of Explant Responses to STING Ligands: Personalized Immunosurgical Therapy for Head and Neck Squamous Cell Carcinoma. Cancer Res. 2018, 78, 6308–6319. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Mozumdar, S.; Huang, L. Lipid-based systemic delivery of siRNA. Adv. Drug Deliv. Rev. 2009, 61, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, E.W. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhao, L.; Hu, H.; Li, W.; Li, Y.-M. Agonists and inhibitors of the STING pathway: Potential agents for immunotherapy. Med. Res. Rev. 2019, 40, 1117–1141. [Google Scholar] [CrossRef]
- Watkins-Schulz, R.; Tiet, P.; Gallovic, M.D.; Junkins, R.D.; Batty, C.; Bachelder, E.M.; Ainslie, K.M.; Ting, J.P.Y. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity. Biomaterials 2019, 205, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhou, X.; Jiang, Z. cGAS-cGAMP-STING: The three musketeers of cytosolic DNA sensing and signaling. IUBMB Life 2016, 68, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cheng, H.; Yuan, H.; Xu, Q.; Shu, C.; Zhang, Y.; Xu, P.; Tan, J.; Rui, Y.; Li, P.; et al. Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci. Rep. 2016, 6, srep19049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulen, M.F.; Koch, U.; Haag, S.M.; Schuler, F.; Apetoh, L.; Villunger, A.; Radtke, F.; Ablasser, A. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 2017, 8, 427. [Google Scholar] [CrossRef]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a Vascular and Pulmonary Syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Konno, H.; Ahn, J.; Barber, G.N. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates with Tumorigenesis. Cell Rep. 2016, 14, 282–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Konno, H.; Barber, G.N. Recurrent Loss of STING Signaling in Melanoma Correlates with Susceptibility to Viral Oncolysis. Cancer Res. 2016, 76, 6747–6759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Queiroz, N.M.G.P.; Xia, T.; Konno, H.; Barber, G.N. Ovarian Cancer Cells Commonly Exhibit Defective STING Signaling Which Affects Sensitivity to Viral Oncolysis. Mol. Cancer Res. 2018, 17, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, P.; Tang, Z.; Zhao, J.; Wu, W.; Li, H.; Shao, M.; Li, L.; Yang, C.; Duan, F.; et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci. Rep. 2017, 7, 39858. [Google Scholar] [CrossRef] [PubMed]
- Edeczkowska, A.; Ebaruch, K.; Eschwartz, M. Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol. 2016, 37, 181–192. [Google Scholar] [CrossRef]
| Small Molecule STING Agonist | hSTING Activity | mSTING Activity | Assay Used | Pertinent Findings | References |
|---|---|---|---|---|---|
| DMXAA | No | Yes | ITC | mSTING: Kd~130 nM. hSTING Kd: undetectable. ITC upper bound of detection ~100 uM therefore hSTING affinity for DMXAA >1000-fold lower than for mSTING. | [62] |
| FAA | No | Yes | Vesicular stomatitis viral inhibition assay | Murine splenic leukocytes generated 250 units/mL * of IFN following 3 h incubation with 0.25 mg/mL FAA compared with <5 units/mL produced in human peripheral blood leukocytes. | [82] |
| CMA | No | Yes | ELISA | CMA in murine model: strong induction of Type-1 IFN production (~1.2 pg/mL after 18 h). CMA in human cells (PBMCs, fibroblasts): failed to induce detectable cytokine responses even at 4000 ug/mL. | [83] |
| α-Mangostin | Yes | Yes | q-RT-PCR + IFN-ß-luciferase reporter | THP1 cells treated with 25uM α-Mangostin for 9 h significantly increased IFN-ß mRNA expression ~8-fold. HEK 293 T cells were transiently transfected with hSTING or mSTING and then transfected with up to 25 uM a-Mangostin. After 24 h, IFN- ß luciferase activity was reported in both mSTING and hSTING but was ~5-fold greater in hSTING. | [84] |
| BNBC | Yes | No | q-RT-PCR | BNBC concentration-dependently induced IFN-ß in HepG2/STING (reconstituted cell line with hSTING) cells but not HepG2/mSTING (reconstituted cell line with mSTING) cells. 200 uM of BNBC significantly induced IFN- ß mRNA expression ~5000-fold in HepG2/STING cells compared with only ~2-fold in HepG2/mSTING cells. | [85] |
| DSDP | Yes | No | q-RT-PCR | DSDP concentration-dependently induced IFN-ß in HepG2/STING cells but not HepG2/mSTING cells. 50uM of DSDP significantly induced IFN-ß mRNA expression ~300-fold in HepG2/STING cells compared with only ~1-fold with no significant difference compared to the internal control. | [86] |
| diABZI | Yes | Yes | IFN- ß secretion assay | In human PBMCs, diABZI induced dose-dependent activation of STING and secretion of IFNβ with an EC50 of 130 nM. This is more than 400-fold more potent than cGAMP. No information on mSTING activity. diABZI activated secretion of Type I IFNs and pro-inflammatory cytokines in wild type but not mice deficient in STING. | [91] |
| Bicyclic benzamides | Yes | No | Luciferase assay | All compounds have a micromolar range of activity in the HEK293T-hSTING luciferase assay, and potently induce the secretion of IFN- β, IL-6, TNF-αand CXCL10 in PBMCs and in BALB/c mice bearing CT26 hSTING expressing tumours. | [88,89,90] |
| Benzothiophenes | Yes | No | 3H-cGAMP filtration binding assay | Of the 5 Benzothiophene derivatives developed by Merck Sharp and Dohme Corporation, 3 compounds show significant functional activity with percent activation (% effect) several folds higher than 2′3′-cGAMP in IFN- ß secretion of THP1 cells.
| [92] |
| MSA-2 | Yes | Yes | AlphaLISA + competitive radioligand binding assay | EC50 of 8.3 and 24 μM for human STING isoforms WT and HAQ, respectively. MSA-2 shows antitumor activity and stimulates interferon-β secretion in tumours, induces tumour regression with durable antitumor immunity, and synergizes with anti-PD-1 in the LL-2 tumour model. It exhibits dose-dependent antitumor activity when administered by IT, SC, or PO routes, and dosing regimens were identified that induced complete tumour regressions in 80 to 100% of treated animals. MSA-2 (PO: 60 mg/kg or SC: 50 mg/kg; single dose) effectively inhibited tumour growth induced substantial elevations of IFN-β, interleukin-6 (IL-6), and TNF-α in MC38 mouse tumour model. Stepwise reductions of extracellular pH from 7.5 to 6 increased MSA-2 potency in both THP-1 cells and mouse macrophages, potency of cGAMP was unchanged with pH changes. | [93] |
| SR-717 | Yes | Yes | q-RT-PCR | Cell based activity of SR-717: ISG-THP1, EC50 = 2.1 μM; ISG-THP1 cGAS KO, EC50 = 2.2 μM; ISG-THP1 STING KO, no activity up to the limit of solubility. SR-717 binds to STING with an apparent affinity IC50 = 7.8 μM. 30 mg/kg intraperitoneal once-per-day regimen of SR-717 for 1 week maximally inhibited tumour growth and prolonged survival in B16F10 model. The compound increased CD8+ T cells among TILs and in dLNs, as well as activated NK cells in dLN. SR-717 induced PD-L1 expression in THP1 cells and in primary human PBMCs. SR-717 STING agonist was found to induce IDO1 expression in primary human PBMCs. | [94] |
| Drug | Company | Cancer Type | Phase | Trial Start Date | Status (Estimated Completion) | Pertinent Findings of Trial | NCT Code |
|---|---|---|---|---|---|---|---|
| ADU-S100 (i.t.) +/− ipilimumab (i.v.) | Aduro Biotech; Novartis | Advanced/metastatic solid tumours; lymphomas | I | 04/16 | Terminated 12/19 | Undisclosed | NCT02675439 |
| ADU-S100 (i.t.) + PDR001(i.v.) (spartalizumab) | Novartis | Solid tumours; lymphomas | Ib | 09/17 | Terminated 12/19 | Data cut-off: 5th April 2019
| NCT03172936 |
| ADU-CL-20 (i.t.) + anti-PD-1 (i.v.) | Aduro Biotech | Metastatic/recurrent HNSCC | II | 08/19 | Ongoing (2022) | Undisclosed | NCT03937141 |
| MK-1454 (i.t.) +/− pembrolizumab (i.v.) | Merck & Co | Advanced/metastatic solid tumours; lymphomas | I | 02/17 | Ongoing (2021) | Data cut-off: 31st July 2018
| NCT03010176 |
| MK-2118 (i.t.; s.c.) +/− pembrolizumab (i.v.) | Merck & Co | Advanced/metastatic solid tumours; lymphomas | I | 09/17 | Ongoing (2022) | Undisclosed | NCT03249792 |
| BMS-986301 (i.t.) +/− nivolumab (i.v.), ipilimumab (i.v.) | Bristol-Myers Squibb | Advanced solid tumours | I | 03/19 | Ongoing (2023) | Undisclosed | NCT03956680 |
| GSK3745417 (i.v.; s.c.) +/− pembrolizumab (i.v.) | GSK | Advanced solid tumours | I | 03/19 | Ongoing (2024) | Undisclosed | NCT03843359 |
| SB-11285 (i.v.) + nivolumab (i.v.) | Spring Bank Pharmaceuticals | Advanced solid tumours | Ia/Ib | 09/19 | Ongoing (2022) | Undisclosed | NCT04096638 |
| IMSA-101 (i.t.) +/− ICI (i.v.) | ImmuneSensor Therapeutics | Advanced solid tumours | I/IIa | 09/19 | Ongoing (2023) | Undisclosed | NCT04020185 |
| E7766 (i.t.) | Eisai Inc. | Advanced solid tumours; lymphomas | Ia/Ib | 03/20 | Ongoing (2022) | Undisclosed | NCT04144140 |
| Drug Delivery System | Loaded CDN | Tumour Models | ROA | Date | References | |
|---|---|---|---|---|---|---|
| YSK05 (pH sensitive cationic lipid with high fusogenicity) | c-di-GMP | B16-F10 (melanoma) | i.v. | 08/15 | [128] | |
| Liposomes | YSK05 (pH sensitive cationic lipid with high fusogenicity) | c-di-GMP | E.G7-OVA (T cell lymphoma) | s.c. | 04/14 | [129] |
| PEGylated lipid nanoparticles | c-di-GMP | EG.7-OVA (T cell lymphoma); B16-F10 (melanoma) | s.c. | 05/15 | [130] | |
| PEGylated cationic liposomes | 2’3’-cGAMP | B16-F10 (melanoma) | i.v.; i.t. | 01/17 | [131] | |
| Soy-PC-DOTAP liposome | 3’3’-cGAMP | C3(1) Tag model (basal-like TNBC); B16F10 (melanoma); C3(1) Tag GEM (basal-like TNBC) | i.v. | 11/2018 | [124] | |
| poly (beta-amino ester) (PBAE) * | ML-RR-S2-CDA (ADU-S100) | B16-F10 (melanoma) | i.t. | 11/17 | [123] | |
| Polymers | PEG-DBP copolymers * | 2’3’-cGAMP | B16-F10 (melanoma) | i.v.; i.t. | 1/19 | [14] |
| PEG-DBP copolymers * | 2’3’-cGAMP | Neuroblastoma | i.t. | 03/20 | [132] | |
| Ace-DEX microparticles | 3’3’-cGAMP | E0771 (TNBC); B16-F10 (melanoma) | i.p.; i.m; i.v.; i.t. | 06/19 | [133] | |
| LPEI/HA | 2’3’-cGAMP;3’3’-cGAMP | N/A | i.m. | 10/15 | [134] | |
| Hydrogels | HA hydrogel scaffold | 2’3’-cGAMP | 4T1 (breast cancer) | i.v.; i.t. | 03/18 | [135] |
| Matrigel | CDA | TC1 (lung cancer) | i.t. | 11/18 | [136] | |
| STINGel | ML-RR-S2-CDA (ADU-S100) | MOC2-E6E7 (Oral cancer) | i.t. | 01/18 | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motedayen Aval, L.; Pease, J.E.; Sharma, R.; Pinato, D.J. Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy. J. Clin. Med. 2020, 9, 3323. https://doi.org/10.3390/jcm9103323
Motedayen Aval L, Pease JE, Sharma R, Pinato DJ. Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy. Journal of Clinical Medicine. 2020; 9(10):3323. https://doi.org/10.3390/jcm9103323
Chicago/Turabian StyleMotedayen Aval, Leila, James E. Pease, Rohini Sharma, and David J. Pinato. 2020. "Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy" Journal of Clinical Medicine 9, no. 10: 3323. https://doi.org/10.3390/jcm9103323
APA StyleMotedayen Aval, L., Pease, J. E., Sharma, R., & Pinato, D. J. (2020). Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy. Journal of Clinical Medicine, 9(10), 3323. https://doi.org/10.3390/jcm9103323






