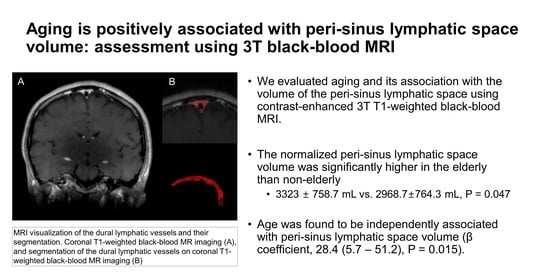
Aging Is Positively Associated with Peri-Sinus Lymphatic Space Volume: Assessment Using 3T Black-Blood MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition
2.3. Image Analyses
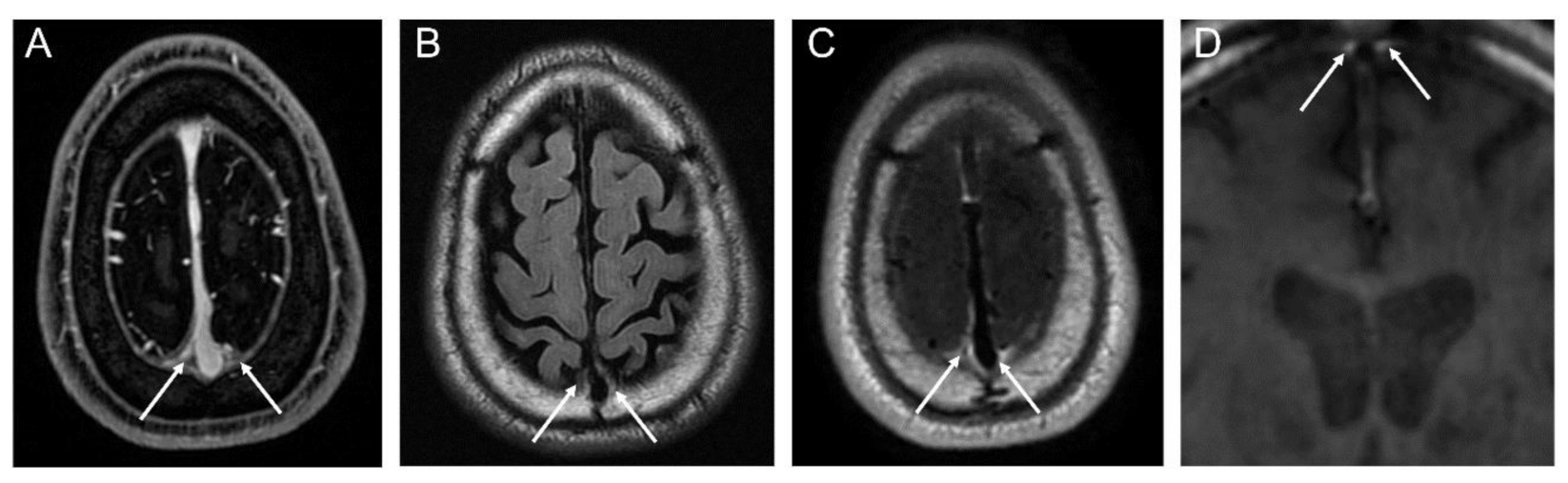
2.4. Segmentation of Dura-Associated Lymphatics
2.5. Brain Volume Analysis
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Aging and Its Association with Normalized Peri-Sinus Lymphatic Volume
3.3. Differences Regarding Effects of Aging on Normalized Peri-Sinus Lymphatic Volume Based on Sex
3.4. Interrater Reliability of Peri-Sinus Lymphatic Space Volume Measurements
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hasek, M.; Chutna, J.; Sladecek, M.; Lodin, Z. Immunological tolerance and tumor allografts in the brain. Nature 1977, 268, 68–69. [Google Scholar] [CrossRef]
- Ringstad, G.; Valnes, L.M.; Dale, A.M.; Pripp, A.H.; Vatnehol, S.S.; Emblem, K.E.; Mardal, K.A.; Eide, P.K. Brain-wide glymphatic enhancement and clearance in humans assessed with mri. JCI Insight 2018, 3, e121537. [Google Scholar] [CrossRef] [Green Version]
- Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal. Fluid Res. 2010, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Ha, S.K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by mri. eLife 2017, 6, e29738. [Google Scholar] [CrossRef]
- Naganawa, S.; Ito, R.; Taoka, T.; Yoshida, T.; Sone, M. The space between the pial sheath and the cortical venous wall may connect to the meningeal lymphatics. Magn. Reson. Med. Sci. 2020, 19, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ringstad, G.; Eide, P.K. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun. 2020, 11, 354. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. Mr signal abnormalities at 1.5 t in alzheimer’s dementia and normal aging. AJR. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Moon, Y.; Han, S.H.; Kim, H.K.; Moon, W.J. Myelin loss in white matter hyperintensities and normal-appearing white matter of cognitively impaired patients: A quantitative synthetic magnetic resonance imaging study. Eur. Radiol. 2019, 29, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Maclullich, A.M.; Wardlaw, J.M.; Ferguson, K.J.; Starr, J.M.; Seckl, J.R.; Deary, I.J. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.H.; Stuehm, C.; Squire, S.; Johnson, K. Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography 2018, 4, 99–104. [Google Scholar]
- Sanfilipo, M.P.; Benedict, R.H.; Zivadinov, R.; Bakshi, R. Correction for intracranial volume in analysis of whole brain atrophy in multiple sclerosis: The proportion vs. Residual method. Neuroimage 2004, 22, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Manjon, J.V.; Coupe, P. Volbrain: An online mri brain volumetry system. Front. Neuroinform. 2016, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Fleiss, J.L. Design and Analysis of Clinical Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 73. [Google Scholar]
- Sommer, N.N.; Pons Lucas, R.; Coppenrath, E.; Kooijman, H.; Galie, F.; Hesse, N.; Sommer, W.H.; Treitl, K.M.; Saam, T.; Froelich, M.F. Contrast-enhanced modified 3d t1-weighted tse black-blood imaging can improve detection of infectious and neoplastic meningitis. Eur. Radiol. 2020, 30, 866–876. [Google Scholar] [CrossRef]
- Lee, K.Y.; Suh, S.H.; Ahn, S.J. Significance of hyperintense arteries on gd-enhanced 3d t1w black-blood imaging in acute stroke. Eur. Radiol. 2019, 29, 1329–1337. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Scholl, J.; Uecker, M.; Detmar, M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.; Berezuk, C.; McNeely, A.A.; Scott, C.J.; Gao, F.; Black, S.E. Visible virchow-robin spaces on magnetic resonance imaging of alzheimer’s disease patients and normal elderly from the sunnybrook dementia study. J. Alzheimers Dis. 2015, 43, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Melbourne, A.; Owen, D.; Cardoso, M.J.; Sudre, C.H.; Tillin, T.; Sokolska, M.; Atkinson, D.; Chaturvedi, N.; Ourselin, S.; et al. Cortical cerebral blood flow in ageing: Effects of haematocrit, sex, ethnicity and diabetes. Eur. Radiol. 2019, 29, 5549–5558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.C.; Tzourio, C.; Soumare, A.; Mazoyer, B.; Dufouil, C.; Chabriat, H. Severity of dilated virchow-robin spaces is associated with age, blood pressure, and mri markers of small vessel disease: A population-based study. Stroke 2010, 41, 2483–2490. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Singh, M. Sex differences in cognitive impairment and alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.A.; Choudhury, K.R.; Rathakrishnan, B.G.; Marks, D.M.; Petrella, J.R.; Doraiswamy, P.M.; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 2015, 1, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roof, R.L.; Hall, E.D. Gender differences in acute cns trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000, 17, 367–388. [Google Scholar] [CrossRef]
- Smirniotopoulos, J.G.; Murphy, F.M.; Rushing, E.J.; Rees, J.H.; Schroeder, J.W. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007, 27, 525–551. [Google Scholar] [CrossRef]
- Sharma, R.; Wendt, J.A.; Rasmussen, J.C.; Adams, K.E.; Marshall, M.V.; Sevick-Muraca, E.M. New horizons for imaging lymphatic function. Ann. N. Y. Acad. Sci. 2008, 1131, 13–36. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Zhang, L.; Ding, G.; Davoodi-Bojd, E.; Li, Q.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow. Metab. 2017, 37, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cai, J.; Zhang, W.; Gong, X.; Yan, S.; Zhang, K.; Luo, Z.; Sun, J.; Jiang, Q.; Lou, M. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann. Neurol. 2020, 87, 357–369. [Google Scholar] [CrossRef] [PubMed]



| Variable | Total Patients n = 165 | Non-Elderly n = 93 | Elderly n = 72 | p Value |
|---|---|---|---|---|
| Sex (M:F) | 91:74 | 45:48 | 46: 26 | 0.058 |
| Age (y) | 62.1 ± 10.9 | 54.6 ± 7.7 | 71.8 ± 5.2 | <0.001 |
| Underlying disease | 0.028 | |||
| Lung cancer | 115 (69.7%) | 57 (61.3%) | 58 (80.6%) | |
| Breast cancer | 20 (12.1%) | 16 (17.2%) | 4 (5.6%) | |
| Other | 20 (12.1%) | 12 (12.9%) | 8 (11.1%) | |
| None | 10 (6.1%) | 8 (8.6%) | 2 (2.8%) | |
| Hypertension | 48 (29.1%) | 15 (16.1%) | 33 (45.8%) | <0.001 |
| Diabetes mellitus | 36 (21.8%) | 17 (18.3%) | 19 (26.4%) | 0.144 |
| Previous chemotherapy | 61 (37.0%) | 31 (33.3%) | 30 (41.7%) | 0.271 |
| Total Fazekas score | 2.1 ± 1.4 | 1.5 ± 0.9 | 2.9 ± 1.6 | <0.001 |
| ePVS-BG (+) | 18 (10.9%) | 3 (3.2%)18 | 15 (20.8%) | <0.001 |
| ePVS-CSO (+) | 60 (36.4%) | 21 (22.6%) | 39 (54.9%) | <0.001 |
| Intracranial volume (cm3) | 1401.1 ± 131.2 | 1402.0 ± 140.3 | 1399.9 ± 119.3 | 0.918 |
| Normalized dural lymphatic volume (mm3) | 4028.0 ± 1529.8 | 3726.5 ± 1513.9 | 4417.6 ± 1470.6 | 0.004 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| β Coefficient | p-Value | β Coefficient | p-Value | |
| Age | 34.0 (12.9–55.1) | 0.002 | 28.4 (5.7–51.2) | 0.015 |
| Male sex | 1158.1 (719.0–1597.3) | <0.001 | 672.4 (109.9–1234.9) | 0.019 |
| DM | 197.9 (−372.3 to 768.3) | 0.494 | - | - |
| HTN | 469.3 (−44.9 to 983.5) | 0.073 | −2.8 (−517.6 to 512.0) | 0.991 |
| ePVS-CSO (+) | 510.2 (26.8–993.6) | 0.039 | 0.415 (−490.1 to 491.0) | 0.999 |
| ePVS-BG (+) | 522.5 (−229.8 to 1274.8) | 0.172 | - | - |
| Intracranial volume (cm3) | 3.9 (2.2–5.6) | <0.001 | 2.3 (0.16–4.4) | 0.035 |
| Brain metastasis | −86.3 (−570.4 to 397.4) | 0.724 | - | - |
| Cancer type | ||||
| None | - | - | - | - |
| Lung cancer | −67.4 (−1052.4 to 917.6) | 0.893 | - | - |
| Other cancer | −715.1 (−1771.4 to 341.2) | 0.183 | - | - |
| Previous Chemotherapy | −313.5 (−799.7 to 172.8) | 0.205 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Kim, J.W.; Ahn, S.J.; Cha, Y.J.; Suh, S.H. Aging Is Positively Associated with Peri-Sinus Lymphatic Space Volume: Assessment Using 3T Black-Blood MRI. J. Clin. Med. 2020, 9, 3353. https://doi.org/10.3390/jcm9103353
Park M, Kim JW, Ahn SJ, Cha YJ, Suh SH. Aging Is Positively Associated with Peri-Sinus Lymphatic Space Volume: Assessment Using 3T Black-Blood MRI. Journal of Clinical Medicine. 2020; 9(10):3353. https://doi.org/10.3390/jcm9103353
Chicago/Turabian StylePark, Mina, Jin Woo Kim, Sung Jun Ahn, Yoon Jin Cha, and Sang Hyun Suh. 2020. "Aging Is Positively Associated with Peri-Sinus Lymphatic Space Volume: Assessment Using 3T Black-Blood MRI" Journal of Clinical Medicine 9, no. 10: 3353. https://doi.org/10.3390/jcm9103353