The HLA Variant rs6903608 Is Associated with Disease Onset and Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura in Caucasians
Abstract
:1. Introduction
2. Materials and Methods
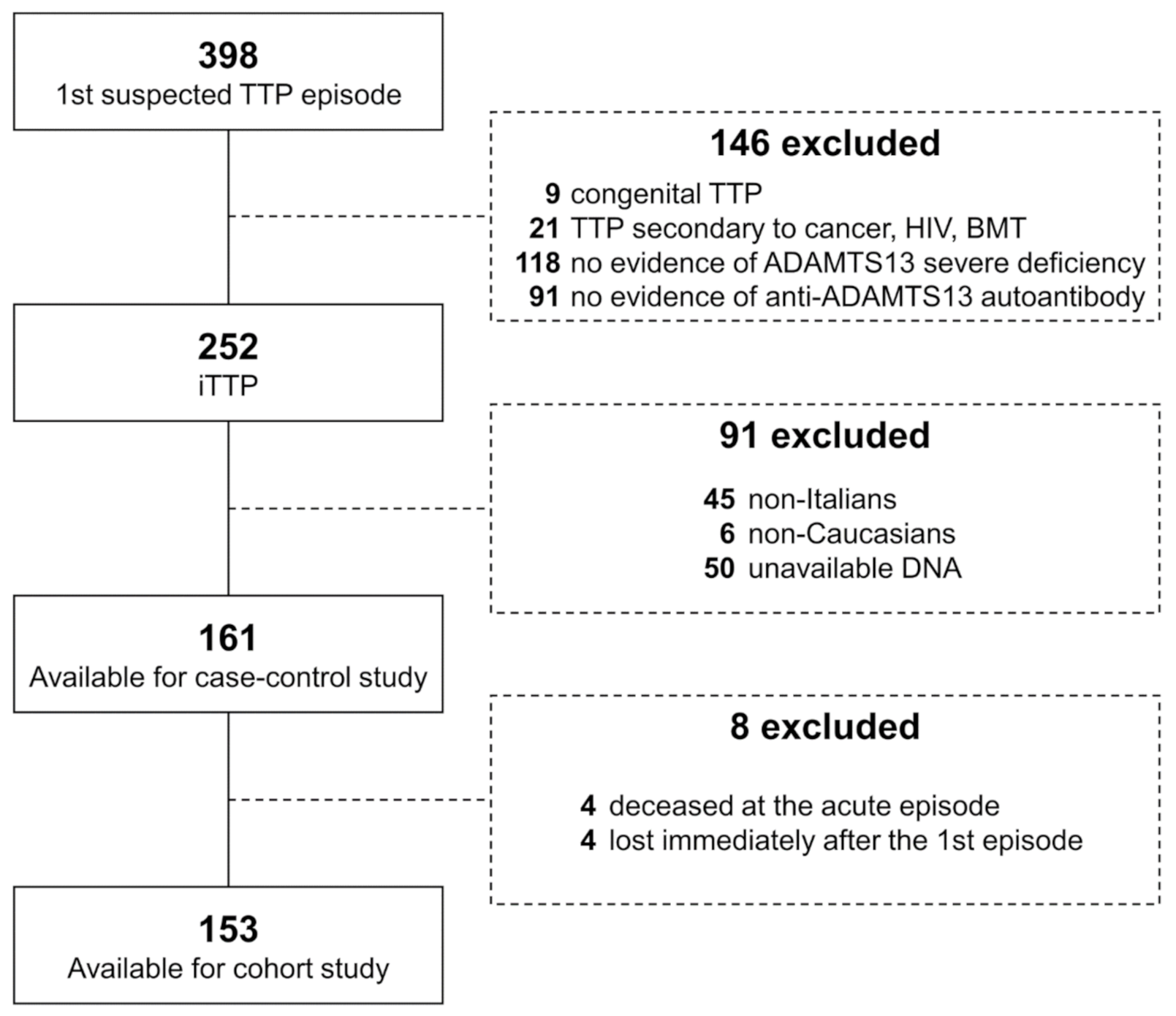
2.1. Study Design, Subjects and Definitions
2.2. rs6903608 Genotyping
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scully, M.; Cataland, S.; Coppo, P.; De La Rubia, J.; Friedman, K.D.; Kremer, H.J.; Lammle, B.; Matsumoto, M.; Pavenski, K.; Sadler, E.; et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J. Thromb. Haemost. 2017, 15, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Reese, J.A.; Muthurajah, D.S.; Kremer Hovinga, J.A.; Vesely, S.K.; Terrell, D.R.; George, J.N. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: Comparison of incidence, demographic and clinical features. Pediatr. Blood Cancer 2013, 60, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Yarranton, H.; Liesner, R.; Cavenagh, J.; Hunt, B.; Benjamin, S.; Bevan, D.; Mackie, I.; Machin, S. Regional UK TTP registry: Correlation with laboratory ADAMTS 13 analysis and clinical features. Br. J. Haematol. 2008, 142, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.; Poullin, P.; De Maistre, E.; Provot, F.; Delmas, Y.; et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): A cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016, 3, e237–e245. [Google Scholar] [CrossRef]
- Deford, C.C.; Reese, J.A.; Schwartz, L.H.; Perdue, J.J.; Kremer Hovinga, J.A.; Lammle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013, 122, 2023–2029. [Google Scholar] [CrossRef] [Green Version]
- Page, E.E.; Kremer Hovinga, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Thrombotic thrombocytopenic purpura: Diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017, 1, 590–600. [Google Scholar] [CrossRef]
- Martino, S.; Jamme, M.; Deligny, C.; Busson, M.; Loiseau, P.; Azoulay, E.; Galicier, L.; Pene, F.; Provot, F.; Dossier, A.; et al. Thrombotic Thrombocytopenic Purpura in Black People: Impact of Ethnicity on Survival and Genetic Risk Factors. PLoS ONE 2016, 11, e0156679. [Google Scholar] [CrossRef] [Green Version]
- Peyvandi, F.; Scully, M.; Kremer Hovinga, J.A.; Knobl, P.; Cataland, S.; De Beuf, K.; Callewaert, F.; De Winter, H.; Zeldin, R.K. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2017, 15, 1448–1452. [Google Scholar] [CrossRef]
- Lotta, L.A.; Mariani, M.; Consonni, D.; Mancini, I.; Palla, R.; Maino, A.; Vucelic, D.; Pizzuti, M.; Mannucci, P.M.; Peyvandi, F. Different clinical severity of first episodes and recurrences of thrombotic thrombocytopenic purpura. Br. J. Haematol. 2010, 151, 488–494. [Google Scholar] [CrossRef]
- Bettoni, G.; Palla, R.; Valsecchi, C.; Consonni, D.; Lotta, L.A.; Trisolini, S.M.; Mancini, I.; Musallam, K.M.; Rosendaal, F.R.; Peyvandi, F. ADAMTS-13 activity and autoantibodies classes and subclasses as prognostic predictors in acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2012, 10, 1556–1565. [Google Scholar] [CrossRef]
- Page, E.E.; Kremer Hovinga, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Clinical importance of ADAMTS13 activity during remission in patients with acquired thrombotic thrombocytopenic purpura. Blood 2016, 128, 2175–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, M.; Brown, J.; Patel, R.; McDonald, V.; Brown, C.J.; Machin, S. Human leukocyte antigen association in idiopathic thrombotic thrombocytopenic purpura: Evidence for an immunogenetic link. J. Thromb. Haemost. 2010, 8, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Busson, M.; Veyradier, A.; Wynckel, A.; Poullin, P.; Azoulay, E.; Galicier, L.; Loiseau, P. HLA-DRB1*11: A strong risk factor for acquired severe ADAMTS13 deficiency-related idiopathic thrombotic thrombocytopenic purpura in Caucasians. J. Thromb. Haemost. 2010, 8, 856–859. [Google Scholar] [CrossRef]
- John, M.L.; Hitzler, W.; Scharrer, I. The role of human leukocyte antigens as predisposing and/or protective factors in patients with idiopathic thrombotic thrombocytopenic purpura. Ann. Hematol. 2012, 91, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Sinkovits, G.; Szilagyi, A.; Farkas, P.; Inotai, D.; Szilvasi, A.; Tordai, A.; Razso, K.; Reti, M.; Prohaszka, Z. The role of human leukocyte antigen DRB1-DQB1 haplotypes in the susceptibility to acquired idiopathic thrombotic thrombocytopenic purpura. Hum. Immunol. 2017, 78, 80–87. [Google Scholar] [CrossRef]
- Mancini, I.; Ricano-Ponce, I.; Pappalardo, E.; Cairo, A.; Gorski, M.M.; Casoli, G.; Ferrari, B.; Alberti, M.; Mikovic, D.; Noris, M.; et al. Immunochip analysis identifies novel susceptibility loci in the human leukocyte antigen region for acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2016, 14, 2356–2367. [Google Scholar] [CrossRef]
- Verbij, F.C.; Turksma, A.W.; De Heij, F.; Kaijen, P.; Lardy, N.; Fijnheer, R.; Sorvillo, N.; ten Brinke, A.; Voorberg, J. CD4+ T cells from patients with acquired thrombotic thrombocytopenic purpura recognize CUB-2 domain derived peptides. Blood 2016, 127, 1606–1609. [Google Scholar] [CrossRef] [Green Version]
- Gilardin, L.; Delignat, S.; Peyron, I.; Ing, M.; Lone, Y.C.; Gangadharan, B.; Michard, B.; Kherabi, Y.; Sharma, M.; Pashov, A.; et al. The ADAMTS13(1239-1253) peptide is a dominant HLA-DR1-restricted CD4(+) T-cell epitope. Haematologica 2017, 102, 1833–1841. [Google Scholar] [CrossRef] [Green Version]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Celius, E.G.; Harbo, H.F.; Egeland, T.; Vartdal, F.; Vandvik, B.; Spurkiand, A. Sex and age at diagnosis are correlated with the HLA-DR2, DQ6 haplotype in multiple sclerosis. J. Neurol. Sci. 2000, 178, 132–135. [Google Scholar] [CrossRef]
- Harbo, H.F.; Isobe, N.; Berg-Hansen, P.; Bos, S.D.; Caillier, S.J.; Gustavsen, M.W.; Mero, I.L.; Celius, E.G.; Hauser, S.L.; Oksenberg, J.R.; et al. Oligoclonal bands and age at onset correlate with genetic risk score in multiple sclerosis. Mult. Scler. 2014, 20, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, K.; Kuwana, M.; Tanaka, H.; Hosomichi, K.; Hasegawa, A.; Uyama, H.; Nishio, K.; Omae, T.; Hishizawa, M.; Matsui, M.; et al. HLA loci predisposing to immune TTP in Japanese: Potential role of the shared ADAMTS13 peptide bound to different HLA-DR. Blood 2020, 135, 2413–2419. [Google Scholar] [CrossRef]

| Variable | Value (n = 161) |
|---|---|
| Female sex, n (%) | 124 (77) |
| Age at first iTTP episode, median (IQR) | 45 (33 to 54) |
| Clinical characteristics | |
| Concomitant autoimmune disease, n (%) * | 19 (12) |
| Infectious trigger, n (%) | 37 (24) |
| Platelet count, ×109/L, median (IQR) † | 13 (8 to 20) |
| Hemoglobin, g/dL, median (IQR) † | 8.0 (6.9 to 9.6) |
| LDH, IU/L, median (IQR) † | 1418 (748 to 2340) |
| rs6903608 Genotype | Cases (n = 161) | Controls (n = 456) | OR (95% CI) |
|---|---|---|---|
| Genotypic model | |||
| TT, n (%) | 20 (12) | 119 (26) | Reference |
| CT, n (%) | 67 (42) | 243 (53) | 1.64 (0.95 to 2.83) |
| CC, n (%) | 74 (46) | 94 (21) | 4.68 (2.67 to 8.23) |
| Dominant model | |||
| TT, n (%) | 20 (12) | 119 (26) | Reference |
| CT + CC, n (%) | 141 (88) | 337 (74) | 2.49 (1.49 to 4.16) |
| Recessive model | |||
| TT + CT, n (%) | 87 (54) | 362 (79) | Reference |
| CC, n (%) | 74 (46) | 94 (21) | 3.28 (2.23 to 4.81) |
| Sex | rs6903608 Genotype | Cases (n = 161) | Controls (n = 456) | OR (95%CI) |
|---|---|---|---|---|
| Male, n (%) | TT | 2 (1) | 27 (6) | Reference |
| CT | 14 (9) | 71 (16) | 2.66 (0.57 to 12.50) | |
| CC | 21 (13) | 22 (5) | 12.89 (2.72 to 61.07) | |
| Female, n (%) | TT | 18 (11) | 92 (20) | 2.64 (0.58 to 12.11) |
| CT | 53 (33) | 172 (38) | 4.16 (0.96 to 18.07) | |
| CC | 53 (33) | 72 (16) | 9.94 (2.26 to 43.63) |
| Concomitant Autoimmune Disorder | Infectious Trigger * | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absent (n = 142) | Present (n = 19) | Absent (n = 119) | Present (n = 37) | Controls (n = 456) | |||||
| rs6903608 | n (%) | OR (95%CI) | n (%) | OR (95%CI) | n (%) | OR (95% CI) | n (%) | OR (95%CI) | n (%) |
| TT | 14 | Ref. | 6 | Ref. | 16 | Ref. | 4 | Ref. | 119 (26) |
| CT | 61 | 2.13 (1.15 to 3.97) | 6 | 0.49 (0.16 to 1.55) | 50 | 1.53 (0.84 to 2.80) | 14 | 1.71 (0.55 to 5.32) | 243 (53) |
| CC | 67 | 6.06 (3.21 to 11.45) | 7 | 1.48 (0.48 to 4.54) | 53 | 4.19 (2.25 to 7.80) | 19 | 6.01 (1.98 to 18.28) | 94 (21) |
| rs6903608 Genotype | Age at Onset (Mean, Range) | Mean Difference, 95% CI (Years) |
|---|---|---|
| TT (n = 20) | 50 (20 to 76) | Reference |
| CT (n = 67) | 45 (17 to 79) | 4, −3 to 12 |
| CC (n = 74) | 42 (11 to 73) | 7, 0 to 14 |
| All iTTP Patients (n = 153) | TT (n = 18) | CT (n = 66) | CC (n = 69) | |
|---|---|---|---|---|
| Median follow-up time, years (95% CI) * | 4.9 (3.7 to 6.1) | 3.6 (0.0 to 7.5) | 5.8 (5.0 to 6.7) | 4.0 (2.9 to 5.1) |
| Relapse n (%) | 44 (29) | 3 (17) | 13 (20) | 28 (41) |
| Death n (%) | 3 (2) | 2 (12) | 0 | 1 (1) |
| Cancer n (%) | 3 (2) | 0 | 2 (3) | 1 (1) |
| Censored alive at the end of study, n (%) | 92 (60) | 12 (67) | 48 (73) | 32 (46) |
| Censored alive lost to follow-up, n (%) | 11 (7) | 2 (11) | 3 (5) | 6 (9) |
| Cumulative Incidence of Relapse, % (95% CI) | ||||
|---|---|---|---|---|
| rs6903608 Genotype | at 2 Years | at 4 Years | at 6 Years | at End of Study |
| TT (n = 18) | 13 (8 to 22) | 13 (8 to 22) | 30 (23 to 39) | 30 (23 to 39) |
| CT (n = 66) | 12 (8 to 17) | 22 (16 to 29) | 22 (16 to 29) | 33 (23 to 46) |
| CC (n = 69) | 23 (16 to 32) | 46 (36 to 57) | 46 (36 to 57) | 76 (44 to 97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, I.; Giacomini, E.; Pontiggia, S.; Artoni, A.; Ferrari, B.; Pappalardo, E.; Gualtierotti, R.; Trisolini, S.M.; Capria, S.; Facchini, L.; et al. The HLA Variant rs6903608 Is Associated with Disease Onset and Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura in Caucasians. J. Clin. Med. 2020, 9, 3379. https://doi.org/10.3390/jcm9103379
Mancini I, Giacomini E, Pontiggia S, Artoni A, Ferrari B, Pappalardo E, Gualtierotti R, Trisolini SM, Capria S, Facchini L, et al. The HLA Variant rs6903608 Is Associated with Disease Onset and Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura in Caucasians. Journal of Clinical Medicine. 2020; 9(10):3379. https://doi.org/10.3390/jcm9103379
Chicago/Turabian StyleMancini, Ilaria, Elisa Giacomini, Silvia Pontiggia, Andrea Artoni, Barbara Ferrari, Emanuela Pappalardo, Roberta Gualtierotti, Silvia Maria Trisolini, Saveria Capria, Luca Facchini, and et al. 2020. "The HLA Variant rs6903608 Is Associated with Disease Onset and Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura in Caucasians" Journal of Clinical Medicine 9, no. 10: 3379. https://doi.org/10.3390/jcm9103379
APA StyleMancini, I., Giacomini, E., Pontiggia, S., Artoni, A., Ferrari, B., Pappalardo, E., Gualtierotti, R., Trisolini, S. M., Capria, S., Facchini, L., Codeluppi, K., Rinaldi, E., Pastore, D., Campus, S., Caria, C., Caddori, A., Nicolosi, D., Giuffrida, G., Agostini, V., ... Peyvandi, F. (2020). The HLA Variant rs6903608 Is Associated with Disease Onset and Relapse of Immune-Mediated Thrombotic Thrombocytopenic Purpura in Caucasians. Journal of Clinical Medicine, 9(10), 3379. https://doi.org/10.3390/jcm9103379








