Somatic Variant Analysis Identifies Targets for Tailored Therapies in Patients with Vascular Malformations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Extraction, Sequencing and Analysis
3. Results
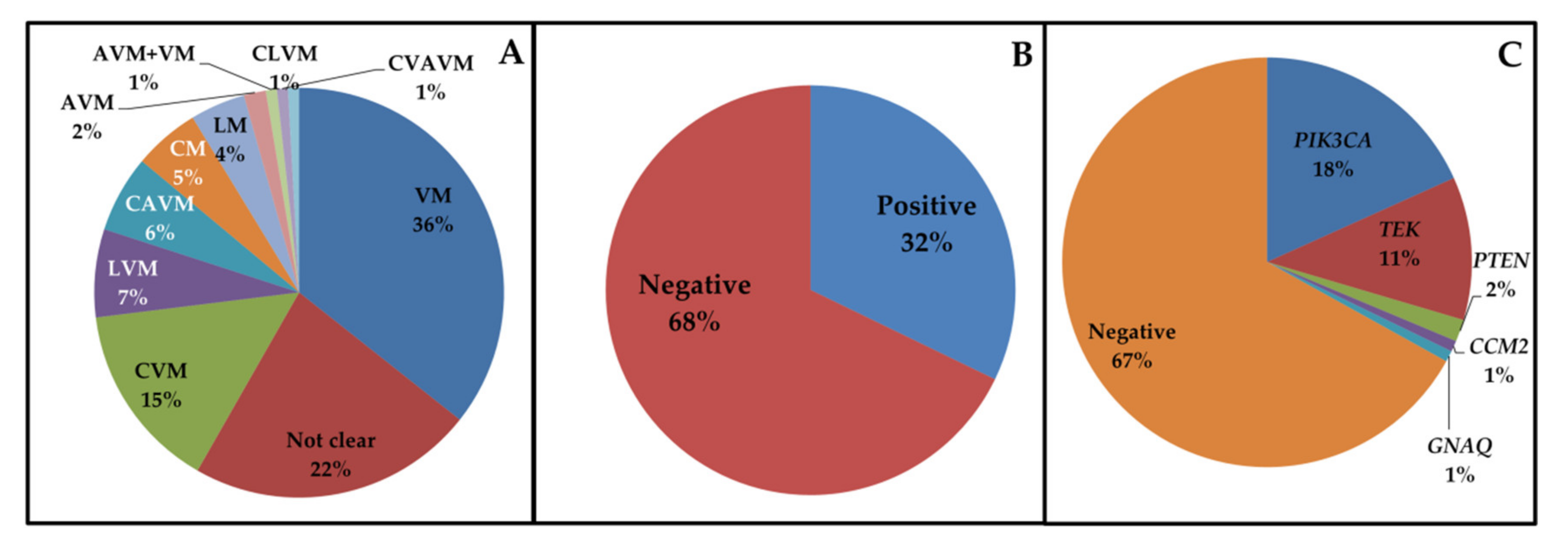
3.1. Cohort Analysis
3.2. PIK3CA Mutations
3.3. TEK Mutations
3.4. Other Mutations
3.5. A Case with Both PTEN and TEK Pathogenic Variants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vikkula, M.; Boon, L.M.; Mulliken, J.B. Molecular Genetics of Vascular Malformations. Matrix Biol. 2001, 20, 327–335. [Google Scholar] [CrossRef]
- ISSVA. ISSVA Classification for Vascular Anomalies (Approved at the 20th ISSVA Workshop, Melbourne, April 2014, Last Revision May 2018). 2018. Available online: http://www.issva.org/UserFiles/file/ISSVA–Classification–2018.pdf (accessed on 10 October 2020).
- Paolacci, S.; Zulian, A.; Bruson, A.; Manara, E.; Michelini, S.; Mattassi, R.E.; Lee, B.B.; Amato, B.; Bertelli, M. Vascular Anomalies: Molecular Bases, Genetic Testing and Therapeutic Approaches. Int. Angiol. 2019, 38, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Laredo, J. Classification of Congenital Vascular Malformations: The Last Challenge for Congenital Vascular Malformations. Phlebology 2012, 27, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge–Weber Syndrome and Port–Wine Stains Caused by Somatic Mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef]
- Rivière, J.B.; Mirzaa, G.M.; O’Roak, B.J.; Beddaoui, M.; Alcantara, D.; Conway, R.L.; St–Onge, J.; Schwartzentruber, J.A.; Gripp, K.W.; Nikkel, S.M.; et al. De Novo Germline and Postzygotic Mutations in AKT3, PIK3R2 and PIK3CA Cause a Spectrum of Related Megalencephaly Syndromes. Nat. Genet. 2012, 44, 934–940. [Google Scholar] [CrossRef]
- Kurek, K.C.; Luks, V.L.; Ayturk, U.M.; Alomari, A.I.; Fishman, S.J.; Spencer, S.A.; Mulliken, J.B.; Bowen, M.E.; Yamamoto, G.L.; Kozakewich, H.P.; et al. Somatic Mosaic Activating Mutations in PIK3CA Cause CLOVES Syndrome. Am. J. Hum. Genet. 2012, 90, 1108–1115. [Google Scholar] [CrossRef]
- Mirzaa, G.; Timms, A.E.; Conti, V.; Boyle, E.A.; Girisha, K.M.; Martin, B.; Kircher, M.; Olds, C.; Juusola, J.; Collins, S.; et al. PIK3CA–Associated Developmental Disorders Exhibit Distinct Classes of Mutations with Variable Expression and Tissue Distribution. JCI Insight 2016, 1, e87623. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.; Kangas, J.; Mendola, A.; Godfraind, C.; Schlögel, M.J.; Helaers, R.; Eklund, L.; Boon, L.M.; Vikkula, M. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am. J. Hum. Genet. 2015, 97, 914–921. [Google Scholar] [CrossRef]
- Orloff, M.S.; He, X.; Peterson, C.; Chen, F.; Chen, J.L.; Mester, J.L.; Eng, C. Germline PIK3CA and AKT1 Mutations in Cowden and Cowden–Like Syndromes. Am. J. Hum. Genet. 2013, 92, 76–80. [Google Scholar] [CrossRef]
- Ye, C.; Pan, L.; Huang, Y.; Ye, R.; Han, A.; Li, S.; Li, X.; Wang, S. Somatic Mutations in Exon 17 of the TEK Gene in Vascular Tumors and Vascular Malformations. J. Vasc. Surg. 2011, 54, 1760–1768. [Google Scholar] [CrossRef]
- Vikkula, M.; Boon, L.M.; Carraway, K.L., 3rd; Calvert, J.T.; Diamonti, A.J.; Goumnerov, B.; Pasyk, K.A.; Marchuk, D.A.; Warman, M.L.; Cantley, L.C.; et al. Vascular Dysmorphogenesis Caused by an Activating Mutation in the Receptor Tyrosine Kinase TIE2. Cell 1996, 87, 1181–1190. [Google Scholar] [CrossRef]
- Wouters, V.; Limaye, N.; Uebelhoer, M.; Irrthum, A.; Boon, L.M.; Mulliken, J.B.; Enjolras, O.; Baselga, E.; Berg, J.; Dompmartin, A.; et al. Hereditary Cutaneomucosal Venous Malformations Are Caused by TIE2 Mutations with Widely Variable Hyper–Phosphorylating Effects. Eur. J. Hum. Genet. 2010, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Nätynki, M.; Kangas, J.; Miinalainen, I.; Sormunen, R.; Pietilä, R.; Soblet, J.; Boon, L.M.; Vikkula, M.; Limaye, N.; Eklund, L. Common and Specific Effects of TIE2 Mutations Causing Venous Malformations. Hum. Mol. Genet. 2015, 24, 6374–6389. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.; Wouters, V.; Uebelhoer, M.; Tuominen, M.; Wirkkala, R.; Mulliken, J.B.; Eklund, L.; Boon, L.M.; Vikkula, M. Somatic Mutations in Angiopoietin Receptor Gene TEK Cause Solitary and Multiple Sporadic Venous Malformations. Nat. Genet. 2009, 41, 118–124. [Google Scholar] [CrossRef]
- Soblet, J.; Kangas, J.; Nätynki, M.; Mendola, A.; Helaers, R.; Uebelhoer, M.; Kaakinen, M.; Cordisco, M.; Dompmartin, A.; Enjolras, O.; et al. Blue Rubber Bleb Nevus (BRBN) Syndrome is Caused by Somatic TEK (TIE2) Mutations. J. Investig. Dermatol. 2017, 137, 207–216. [Google Scholar] [CrossRef]
- Soblet, J.; Limaye, N.; Uebelhoer, M.; Boon, L.M.; Vikkula, M. Variable Somatic TIE2 Mutations in Half of Sporadic Venous Malformations. Mol. Syndromol. 2013, 4, 179–183. [Google Scholar]
- Nelen, M.R.; van Staveren, W.C.; Peeters, E.A.; Hassel, M.B.; Gorlin, R.J.; Hamm, H.; Lindboe, C.F.; Fryns, J.P.; Sijmons, R.H.; Woods, D.G.; et al. Germline Mutations in the PTEN/MMAC1 Gene in Patients with Cowden Disease. Hum. Mol. Genet. 1997, 6, 1383–1387. [Google Scholar] [CrossRef]
- Klebanov, N.; Lin, W.M.; Artomov, M.; Shaughnessy, M.; Njauw, C.N.; Bloom, R.; Eterovic, A.K.; Chen, K.; Kim, T.B.; Tsao, S.S.; et al. Use of Targeted Next–Generation Sequencing to Identify Activating Hot Spot Mutations in Cherry Angiomas. JAMA Dermatol. 2019, 155, 211–215. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Michelhaugh, S.K.; Klinger, N.V.; Kupsky, W.J.; Sood, S.; Chugani, H.T.; Mittal, S.; Juhász, C. GNAQ Mutation in the Venous Vascular Malformation and Underlying Brain Tissue in Sturge–Weber Syndrome. Neuropediatrics 2017, 48, 385–389. [Google Scholar] [CrossRef]
- Eichenfield, D.Z.; Cotter, D.; Thorson, J.; Hinds, B.; Sun, B.K. Agminated Blue Nevus with a GNAQ Mutation: A Case Report and Review of the Literature. J. Cutan. Pathol. 2019, 46, 130–133. [Google Scholar] [CrossRef]
- Mattassi, R.; Manara, E.; Colombo, P.G.; Manara, S.; Porcella, A.; Bruno, G.; Bruson, A.; Bertelli, M. Variant Discovery in Patients with Mendelian Vascular Anomalies by Next–Generation Sequencing and Their Use in Patient Clinical Management. J. Vasc. Surg. 2018, 67, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, H.; Yang, X.; Wang, Y.; Gu, H.; Liu, M.; Lin, X. Risk Factors Associated with Pain in Patients with Venous Malformations of the Extremities. Vasc. Med. 2019, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, C.J.; Barker, F.G. Cranial Cavernous Malformations. Stroke 2018, 49, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R. PTEN Hamartoma Tumor Syndrome: A Clinical Overview. Cancers 2019, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Belov, S. Classification of Congenital Vascular Defects. Int. Angiol. 1990, 9, 141–146. [Google Scholar]
- Bevacqua, M.; Baldo, F.; Pastore, S.; Valencic, E.; Tommasini, A.; Maestro, A.; Rabusin, M.; Arbo, A.; Barbi, E. Off–Label Use of Sirolimus and Everolimus in a Pediatric Center: A Case Series and Review of the Literature. Pediatr. Drugs 2019, 21, 185–193. [Google Scholar] [CrossRef]

| Gene | Disease (ISSVA Classification) |
|---|---|
| PIK3CA | Common (cystic) LM, common VM, Klippel–Trenaunay syndrome, MCAP, CLOVES syndrome, CLAPO syndrome, FAVA syndrome |
| TEK | Common VM, familial VM cutaneo–mucosal, blue rubber bleb nevus syndrome |
| GLMN | Glomuvenous malformations |
| MAP3K3 | Verrucous venous malformation |
| KRIT1, CCM2, PDCD10 | Cerebral cavernous malformations |
| GNAQ | Congenital hemangioma, CM “Port–wine” stain, nonsyndromic CM, CM of Sturge–Weber syndrome |
| GNA11 | Congenital hemangioma, CM with bone and/or soft tissue hyperplasia, diffuse CM with overgrowth |
| RASA1 | CM–AVM, Parkes–Weber syndrome |
| MAP2K1 | AVM, AVF |
| AKT1 | Proteus syndrome |
| GNA14 | Tufted angioma, pyogenic granuloma, KHE |
| IDH1, IDH2 | Maffucci syndrome, spindle–cell hemangioma |
| PTEN | Bannayan–Riley–Ruvalcaba syndrome, hamartoma of soft tissue/angiomatosis of soft tissue |
| Gene | ID | Gender | Onset | Variant | rs | MAF (%) | Polyphen-2 | SIFT | Mutation Taster | Allelic Imbalance | Malformation (Description, Site) | Other Features | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIK3CA | Case 1 | M | C | c.241G > A; p.E81K | rs1057519929 | NR | PrD | D | DC | 37% | LVM (Phlebectasia, lymphatic deficit in both legs) | OG (feet), NMD | [6] |
| Case 2 | F | I | c.1035T > A; p.N345K | rs121913284 | NR | PrD | T | DC | 8% | VM (Dilated subcutaneous veins, right leg) | Extra–, intramuscular AH | – | |
| Case 3 | F | C | c.1132T > C; p.C378R | novel | NR | PrD | T | DC | 6% | LVM (right leg) | – | ||
| Case 4 | F | I | c.1258T > C; p.C420R | rs121913272 | NR | PrD | D | DC | 7% | VM (Intramuscular, right upper limb) | – | [7] | |
| Case 5 | M | U | c.1357G > A; p.E453K | rs1057519925 | NR | PoD | T | DC | 6% | VM (Diffuse superficial phlebectasias) | [8] | ||
| Case 6 | F | I | c.1624G > A; p.E542K | rs121913273 | NR | PrD | D | DC | 9% | LM (Intramuscular, left arm) | AH, MR | [9] | |
| Case 7 | F | U | c.1624G > A; p.E542K | rs121913273 | NR | PrD | D | DC | 10% | CVM (Painful extramuscular, left arm) | – | [9] | |
| Case 8 | F | U | c.1633G > A; p.E545K | rs104886003 | 0.0004% | PrD | D | DC | 9% | VM (Subcutaneous intra– and extra muscular, hip and right leg) | – | [6] | |
| Case 9 | F | U | c.1633G > A; p.E545K | rs104886003 | 0.0004% | PrD | D | DC | 5% | VM (Right thigh and elbow) | – | [6] | |
| Case 10 | F | C | c.1633G > A; p.E545K | rs104886003 | 0.0004% | PrD | D | DC | 9% | CVM (Deep intramuscular, left arm) | – | [6] | |
| Case 11 | F | C | c.1633G > A; p.E545K | rs104886003 | 0.0004% | PrD | D | DC | 5% | LM (Painful, right arm) | – | [6] | |
| Case 12 | M | C | c.1633G > A; p.E545K | rs104886003 | 0.0004% | PrD | D | DC | 5% | LVM (Phlebectasia, lymphatic deficit, left limb) | [9] | ||
| Case 13 | M | I | c.1634A > C; p.E545A | rs121913274 | NR | PrD | D | DC | 5% | VM (Chest, left shoulder) | – | [10] | |
| Case 14 | F | I | c.1637A > G; p.Q546R | rs397517201 | NR | PrD | T | DC | 7% | CVM (Left leg) | – | – | |
| Case 15 | F | C | c.2740G > A; p.G914R | rs587776932 | NR | PrD | D | DC | 10% | VM (Right leg, shoulder) | dys | [6] | |
| Case 16 | M | C | c.3062A > G; p.Y1021C | rs121913288 | NR | PrD | D | DC | 6% | LVM (Superficial, left leg) | [6] | ||
| Case 17 | M | U | c.3140A > G; p.H1047R | rs121913279 | 0.0004% | PoD | D | DC | 8% | CVM (Painful, right hand) | – | [9] | |
| Case 18 | M | U | c.3140A > G; p.H1047R | rs121913279 | 0.0004% | PoD | D | DC | 8% | CVM (Intra– and extramuscular, right chest) | AH | [9] | |
| Case 19 | F | I | c.3140A > G; p.H1047R | rs121913279 | 0.0004% | PoD | D | DC | 6% | VM (Intramuscular, right leg) | – | [9] | |
| Case 20 | F | I | c.3140A > G; p.H1047R | rs121913279 | 0.0004% | PoD | D | DC | 5% | LVM (Subcutaneous, left leg) | – | [9] | |
| Case 21 | F | C | c.3140A > G; p.H1047R | rs121913279 | 0.0004% | PoD | D | DC | >20% | CLVM | CLOVES | [9] | |
| TEK | Case 22 | F | C | c.[2545C > T; 2743C > T]; p.[R849W; R915C] | rs80338908; no rs | 0.0004%; NR | PrD; PrD | D; D | DC; DC | 6%;5% | VM (Finger) | – | [11,12] |
| Case 23 | F | I | c.2678A > T; p.E893V | novel | NR | PrD | D | DC | 5% | VM (Mucocutaneous, neck) | – | – | |
| Case 24 | M | I | c.[2690A > G; 2752C > T]; p.[Y897C; R918C] | rs80338909; no rs | NR; NR | PrD; PrD | D; D | DC; DC | 6%; 5% | VM (Laterocervical) | [13,14] | ||
| Case 25 | F | I | c.2740C > T; p.L914F | no rs | NR | PrD | D | DC | 10% | CVM (Painful extra– and intramuscular, shoulder and neck) | [15] | ||
| Case 26 | F | I | c.2740C > T; p.L914F | no rs | NR | PrD | D | DC | 10% | VM (Painful intra– and extramuscular, right hip) | – | [15] | |
| Case 27 | F | U | c.2740C > T; p.L914F | no rs | NR | PrD | D | DC | 8% | VM (Painful, right arm) | AH | [15] | |
| Case 28 | F | I | c.2740C > T; p.L914F | no rs | NR | PrD | D | DC | 10% | CVM (Painful, right hand) | – | [15] | |
| Case 29 | F | I | c.2740C > T; p.L914F | no rs | NR | PrD | D | DC | 10% | VM (Painful, left arm and hand) | – | [15] | |
| Case 30 | F | I | c.2753G > A; p.R918H | rs1554701458 | NR | PrD | D | DC | 6% | VM (Painful intra–and extramuscular, neck) | – | – | |
| Case 31 | F | U | c.3295C > T; p.R1099* | no rs | NR | / | / | DC | 9% | VM (Multiple subcutaneous, right arm) | [16] | ||
| Case 32 | M | C | c.3324T > A; p.Y1108* | no rs | NR | / | / | DC | 8% | VM (Painful intramuscular, left thigh) | – | [16] | |
| Case 33 | F | U | c.3343G > T; p.G1115* | no rs | NR | / | / | DC | 9% | VM (Multiple painful, left hand, elbow, arm, axilla) | – | [17] | |
| PTEN | Case 34 | M | I | c.388C > T; p.R130* | rs121909224 | 0.001% | / | / | DC | 5% | AVM + VM (AVM supraclavicular, VM intramuscular, leg, neck, abdomen) | – | [18] |
| TEK | c.3324T > A; p.Y1108* | no rs | NR | / | / | DC | 8% | [16] | |||||
| GNAQ | Case 35 | F | U | c.626A > G; p.Q209R | rs121913492 | NR | PrD | D | DC | 12% | CVM (Eyebrow) | [19] | |
| CCM2 | Case 36 | M | A | c.164_165del; p.P55Rfs*9 | novel | NR | / | / | DC | 6% | VM (Subcutaneous venous lacunae in right arm, cerebral cavernous angioma) | – | – |
| PTEN | Case 37 | F | I | c.697C > T; p.R233* | rs121909219 | NR | / | / | DC | 15% | VM (Painful extra– and intramuscular, left knee) | – | – |
| Disease | Mutated Genes | Affected Pathways | Potential Drugs (ClinicalTrial.gov ID) |
|---|---|---|---|
| Venous malformation | TEK, PIK3CA | RAS/MAPK, PI3K/AKT | Rapamycin (NCT00975819, NCT03767660), regorafenib (NCT02736305), miransertib (NCT03317366) |
| Cerebral cavernous malformations | KRIT1, CCM2, PDCD10 | PI3K/AKT, RHO/ROCK, RAS/MAPK | Propranolol (NCT03474614, NCT03523650, NCT03589014), simvastatin (NCT01764451), atorvastatin (NCT02603328) |
| Capillary malformations | GNAQ, GNA11 | RAS/MAPK, PI3K/AKT | Rapamycin (NCT00800722), hemoporfin (NCT03181984), bosentan (NCT02317679) |
| Arteriovenous malformations (sporadic, in HHT, in CM–AVM) and arteriovenous fistula | GDF2, MAP2K1, ENG, ACVRL1, SMAD4, RASA1, EPHB4 | PI3K/AKT, TGF–β/BMP, RAS/MAPK | Tamoxifen (NCT00375622), doxycycline hyclate (NCT03397004), tranexamic acid (NCT01031992), marimastat (NCT00261391), rapamycin (NCT02042326), bevacizumab (NCT02314377, NCT01402531, NCT01397695), minocycline/doxycycline (NCT00243893), thalidomide (NCT00389935, NCT00964496), doxycycline (NCT00783523), atorvastatin (NCT03188978) |
| Lymphatic malformations | PIK3CA, FLT4, VEGFC, GJC2, FOXC2, SOX18, GATA2, CCBE1, KIF11, PTPN14 | RAS/MAPK, PI3K/AKT, VEGF/VEGFR | Sildenafil (NCT01290484), picibanil (NCT03427619), rapamycin (NCT04128722), alpelisib (NCT03941782), marimastat (NCT00261391) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolacci, S.; Mattassi, R.E.; Marceddu, G.; Manara, E.; Zulian, A.; Guerri, G.; De Antoni, L.; Arduino, C.; Cavalca, D.; Bertelli, M. Somatic Variant Analysis Identifies Targets for Tailored Therapies in Patients with Vascular Malformations. J. Clin. Med. 2020, 9, 3387. https://doi.org/10.3390/jcm9113387
Paolacci S, Mattassi RE, Marceddu G, Manara E, Zulian A, Guerri G, De Antoni L, Arduino C, Cavalca D, Bertelli M. Somatic Variant Analysis Identifies Targets for Tailored Therapies in Patients with Vascular Malformations. Journal of Clinical Medicine. 2020; 9(11):3387. https://doi.org/10.3390/jcm9113387
Chicago/Turabian StylePaolacci, Stefano, Raul Ettore Mattassi, Giuseppe Marceddu, Elena Manara, Alessandra Zulian, Giulia Guerri, Luca De Antoni, Carlo Arduino, Daniela Cavalca, and Matteo Bertelli. 2020. "Somatic Variant Analysis Identifies Targets for Tailored Therapies in Patients with Vascular Malformations" Journal of Clinical Medicine 9, no. 11: 3387. https://doi.org/10.3390/jcm9113387
APA StylePaolacci, S., Mattassi, R. E., Marceddu, G., Manara, E., Zulian, A., Guerri, G., De Antoni, L., Arduino, C., Cavalca, D., & Bertelli, M. (2020). Somatic Variant Analysis Identifies Targets for Tailored Therapies in Patients with Vascular Malformations. Journal of Clinical Medicine, 9(11), 3387. https://doi.org/10.3390/jcm9113387





