Derivation and Validation of Clinical Prediction Models for Rapid Risk Stratification for Time-Sensitive Management for Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort and Sample
2.2. Outcome and Variable Definitions
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
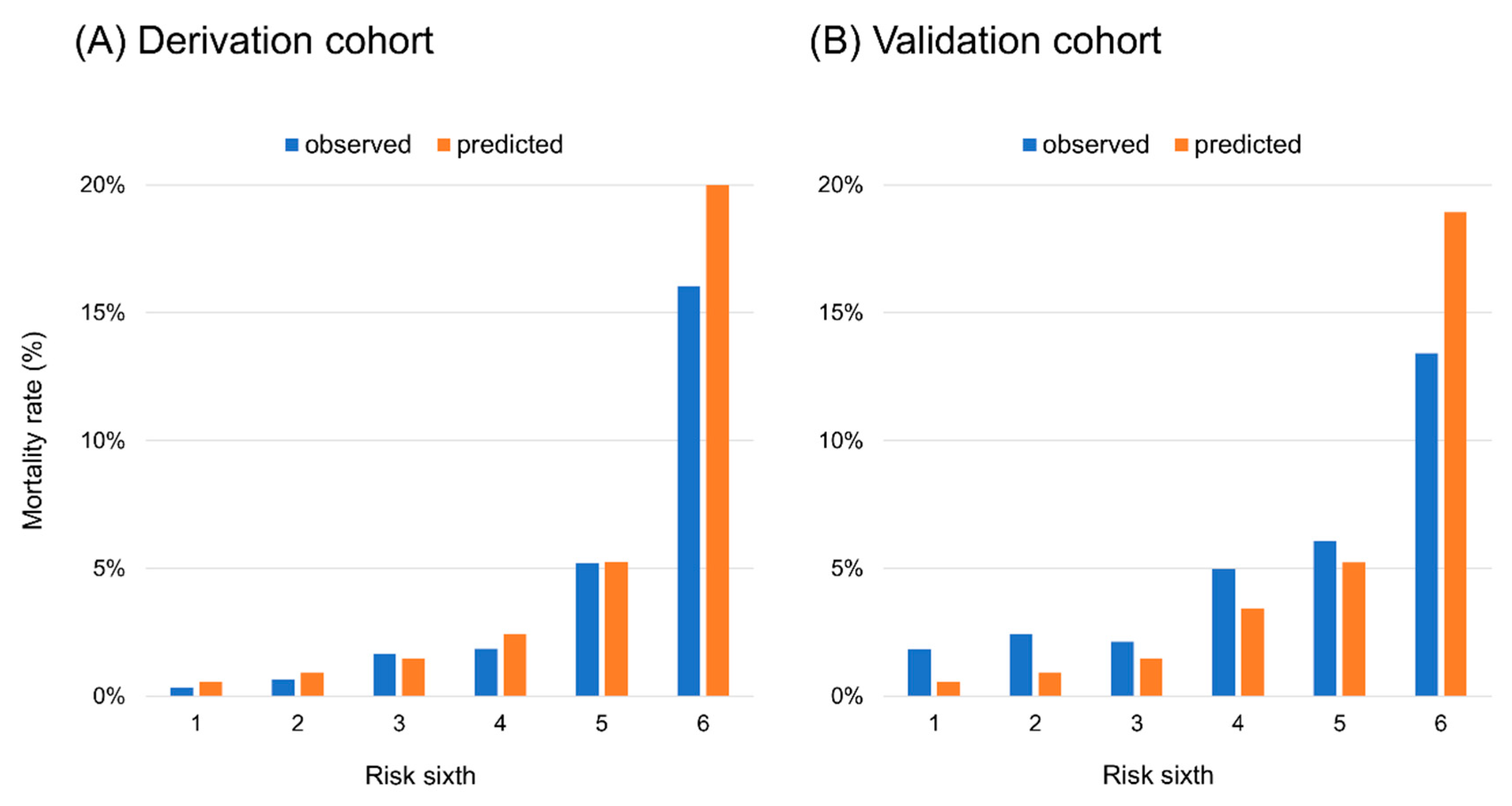
3.2. Model Development and Validation
3.3. Integer Score Calculation and Validation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [PubMed] [Green Version]
- Tsutsui, H.; Isobe, M.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; Komuro, I.; et al. Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure—Digest Version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Stitt, A.; Austin, P.C.; Stukel, T.A.; Schull, M.J.; Chong, A.; Newton, G.E.; Lee, J.S.; Tu, J.V. Prediction of heart failure mortality in emergent care: A cohort study. Ann. Intern. Med. 2012, 156, 767–775. [Google Scholar] [CrossRef]
- Collins, S.P.; Jenkins, C.A.; Harrell, F.E.; Liu, D.; Miller, K.F.; Lindsell, C.J.; Naftilan, A.J.; McPherson, J.A.; Maron, D.J.; Sawyer, D.B.; et al. Identification of emergency department patients with acute heart failure at low risk for 30-day adverse events: The STRATIFY decision tool. JACC Heart Fail. 2015, 3, 737–747. [Google Scholar] [CrossRef]
- Stiell, I.G.; Perry, J.J.; Clement, C.M.; Brison, R.J.; Rowe, B.H.; Aaron, S.D.; McRae, A.D.; Borgundvaag, B.; Calder, L.A.; Forster, A.J.; et al. Prospective and explicit clinical validation of the Ottawa heart failure risk scale, with and without use of quantitative NT-proBNP. Acad. Emerg. Med. 2017, 24, 316–327. [Google Scholar] [CrossRef]
- Miró, Ò.; Rossello, X.; Gil, V.; Martín-Sánchez, F.J.; Llorens, P.; Herrero-Puente, P.; Jacob, J.; Bueno, H.; Pocock, S.J. ICA-SEMES Research Group. Predicting 30-day mortality for patients with acute heart failure in the emergency department: A cohort study. Ann. Intern. Med. 2017, 167, 698–705. [Google Scholar]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kohsaka, S.; Abe, T.; Mizuno, A.; Goda, A.; Izumi, Y.; Yagawa, M.; Akita, K.; Sawano, M.; Inohara, T.; et al. West Tokyo Heart Failure Registry Investigators. Validation of the Get With The Guideline-Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B-type natriuretic peptide level. Am. Heart J. 2016, 171, 33–39. [Google Scholar] [CrossRef]
- Nagai, T.; Nishimura, K.; Honma, T.; Higashiyama, A.; Sugano, Y.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Kawakami, S.; et al. NaDEF investigators. Prognostic significance of endogenous erythropoietin in long-term outcome of patients with acute decompensated heart failure. Eur. J. Heart Fail. 2016, 18, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Matsue, Y.; Damman, K.; Voors, A.A.; Kagiyama, N.; Yamaguchi, T.; Kuroda, S.; Okumura, T.; Kida, K.; Mizuno, A.; Oishi, S.; et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 3042–3051. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef]
- Gupta, A.; Allen, L.A.; Bhatt, D.L.; Cox, M.; DeVore, A.D.; Heidenreich, P.A.; Hernandez, A.F.; Peterson, E.D.; Matsouaka, R.A.; Yancy, C.W.; et al. Association of the Hospital Readmissions Reduction Program Implementation With Readmission and Mortality Outcomes in Heart Failure. JAMA Cardiol. 2018, 3, 44–53. [Google Scholar] [CrossRef]
- Gupta, A.; Fonarow, G.C. The Hospital Readmissions Reduction Program-learning from failure of a healthcare policy. Eur. J. Heart Fail. 2018, 20, 1169–1174. [Google Scholar] [CrossRef] [Green Version]
- Joynt, K.E.; Figueroa, J.E.; Oray, J.; Jha, A.K. Opinions on the Hospital Readmission Reduction Program: Results of a national survey of hospital leaders. Am. J. Manag. Care 2016, 22, e287–e294. [Google Scholar]
- Ibrahim, A.M.; Dimick, J.B.; Sinha, S.S.; Hollingsworth, J.M.; Nuliyalu, U. Ryan AM. Association of Coded Severity With Readmission Reduction After the Hospital Readmissions Reduction Program. JAMA Intern. Med. 2018, 178, 290–292. [Google Scholar] [CrossRef]
- Zuckerman, R.B.; Sheingold, S.H.; Orav, E.J.; Ruhter, J.; Epstein, A.M. Readmissions, Observation, and the Hospital Readmissions Reduction Program. N. Engl. J. Med. 2016, 374, 1543–1551. [Google Scholar] [CrossRef]
- Maisel, A.; Hollander, J.E.; Guss, D.; McCullough, P.; Nowak, R.; Green, G.; Saltzberg, M.; Ellison, S.R.; Bhalla, M.A.; Bhalla, V.; et al. Rapid Emergency Department Heart Failure Outpatient Trial investigtors. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic epptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J. Am. Coll. Cardiol. 2004, 44, 1328–1333. [Google Scholar] [PubMed] [Green Version]
- Lee, D.S.; Lee, J.S.; Schull, M.J.; Borgundvaag, B.; Edmonds, M.L.; Ivankovic, M.; McLeod, S.L.; Dreyer, J.F.; Sabbah, S.; Levy, P.D.; et al. Prospective Validation of the Emergency Heart Failure Mortality Risk Grade for Acute Heart Failure. Circulation 2019, 139, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Sawano, M.; Shiraishi, Y.; Kohsaka, S.; Nagai, T.; Goda, A.; Mizuno, A.; Sujino, Y.; Nagatomo, Y.; Kohno, T.; Anzai, T.; et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018, 5, 610–619. [Google Scholar] [CrossRef]
- Packer, M.; O’Connor, C.; McMurray, J.J.V.; Wittes, J.; Abraham, W.T.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Holcomb, R.; Krum, H.; et al. TRUE-AHF Investigators. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N. Engl. J. Med. 2017, 376, 1956–1964. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Voors, A.A.; et al. RELAX-AHF-2 Committees Investigators. Effects of Serelaxin in Patients with Acute Heart Failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef]
- Pang, P.S.; Butler, J.; Collins, S.P.; Cotter, G.; Davison, B.A.; Ezekowitz, J.A.; Filippatos, G.; Levy, P.D.; Metra, M.; Ponikowski, P.; et al. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: A randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur. Heart J. 2017, 38, 2364–2373. [Google Scholar] [CrossRef] [Green Version]
- Maisel, A.S.; Peacock, W.F.; McMullin, N.; Jessie, R.; Fonarow, G.C.; Wynne, J.; Mills, R.M. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: An ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J. Am. Coll. Cardiol. 2008, 52, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Gul, B.; Bellumkonda, L. Usefulness of Intra-aortic Balloon Pump in Patients With Cardiogenic Shock. Am. J. Cardiol. 2019, 123, 750–756. [Google Scholar] [CrossRef]
- Dangers, L.; Bréchot, N.; Schmidt, M.; Lebreton, G.; Hékimian, G.; Nieszkowska, A.; Besset, S.; Trouillet, J.L.; Chastre, J.; Leprince, P.; et al. Extracorporeal Membrane Oxygenation for Acute Decompensated Heart Failure. Crit. Care Med. 2017, 45, 1359–1366. [Google Scholar] [CrossRef]
- Morganti, K.G.; Bauhoff, S.; Blanchard, J.C.; Abir, M.; Iyer, N.; Smith, A.; Vesely, J.V.; Okeke, E.N.; Kellermann, A.L. The Evolving Role of Emergency Departments in the United States. Rand Health Q. 2013, 3, 3. [Google Scholar]
- Honeyford, K.; Bell, D.; Chowdhury, F.; Quint, J.; Aylin, P.; Bottle, A. Unscheduled hospital contacts after inpatient discharge: A national observational study of COPD and heart failure patients in England. PLoS ONE 2019, 14, e0218128. [Google Scholar] [CrossRef] [PubMed]

| Variable | Derivation Cohort n = 4351 | Validation Cohort n = 1682 |
|---|---|---|
| Background | ||
| Age, years | 74.6 ± 13.1 | 77.5 ± 12.4 * |
| Male, % | 59.9 | 55.4 |
| Body mass index, kg/m2 | 23.2 ± 4.5 | 23.1 ± 4.8 |
| Systolic blood pressure, mm Hg | 140.1 ± 33.6 | 149.0 ± 37.1 * |
| Heart rate, beat per minute | 93.7 ± 29.0 | 97.5 ± 28.3 * |
| Ejection fraction, % | 43.5 ± 15.9 | 46.3 ± 16.0 * |
| NYHA functional class, % | ||
| II | 17.8 | 14.2 † |
| III | 37.8 | 37.5 |
| IV | 44.4 | 48.3 † |
| Ischemic etiology, % | 28.5 | 30.3 |
| Comorbidities | ||
| Prior admissions for heart failure, % | 33.2 | N/A |
| Hypertension, % | 67.7 | 67.3 |
| Hyperlipidemia, % | 41.0 | 37.1 † |
| Diabetes mellitus, % | 35.1 | 36.9 |
| Atrial fibrillation, % | 47.6 | 39.1 * |
| Stroke, % | 16.0 | 11.3 * |
| COPD, % | 5.1 | 9.0 * |
| Laboratory findings at admission | ||
| Hemoglobin, mg/dL | 12.0 ± 2.3 | 11.7 ± 2.3 * |
| Creatinine, mg/dL | 1.1 (0.8–1.5) | 1.1 (0.8–1.6) |
| BUN, mg/dL | 27.6 ± 17.3 | 29.5 ± 17.4 * |
| Sodium, mEq/L | 139.2 ± 4.4 | 138.9 ± 4.7 † |
| Total bilirubin, mg/dL | 1.0 ± 0.7 | 0.9 ± 0.9 † |
| Albumin, mg/dL | 3.6 ± 0.5 | 3.5 ± 0.5 * |
| BNP, pg/mL ‡ | 658 (335–1209) | 744 (444–1343) * |
| NT-proBNP, pg/mL ‡ | 3867 (1917–8741) | 6820 (2908–13,840) * |
| Medication before admission | ||
| Loop diuretics, % | 46.1 | 50.9 † |
| ACEI or ARB, % | 44.1 | 46.2 |
| Beta blocker, % | 44.4 | 43.2 |
| Aldosterone antagonist, % | 18.4 | 22.1 † |
| Digitalis, % | 8.6 | 5.4 * |
| Treatment during admission | ||
| Loop diuretics, IV, % | 68.2 | 82.8 * |
| Vasodilator, IV, % | 61.8 | 60.7 |
| Inotrope, IV, % | 16.1 | 16.1 |
| Non-invasive ventilation, % | 20.1 | 24.2 * |
| Intubation, % | 4.4 | 6.7 * |
| IABP, % | 1.9 | 1.6 |
| VA-ECMO, % | 0.5 | 0.4 |
| Dialysis, % | 4.7 | 3.3 |
| Variable | Total/Missing Data (%) | Wald χ2 | OR (95%CI) | p Value |
|---|---|---|---|---|
| Age (per 1 years) | 4351/0 (100%) | 22.49 | 1.04 (1.02, 1.05) | <0.0001 |
| Male | 4351/0 (100%) | 0.75 | 0.87 (0.64, 1.19) | 0.3852 |
| Body mass index (per 1 kg/m2 increase) | 4026/325 (92.5%) | 35.19 | 0.87 (0.83, 0.91) | <0.0001 |
| SBP (per 1 mm Hg increase) | 4340/11 (99.7%) | 44.40 | 0.98 (0.98, 0.99) | <0.0001 |
| Heart rate (per 1 bpm increase) | 4323/28 (99.4%) | 2.89 | 1.00 (0.99, 1.00) | 0.0890 |
| LVEF (continuous) | 4192/159 (96.3%) | 7.85 | 0.98 (0.97, 1.00) | 0.0051 |
| LVEF (categorical) | 4192/159 (96.3%) | |||
| <20% | 1.00 (reference) | |||
| 20−40% | 13.42 | 0.36 (0.21, 0.62) | 0.0002 | |
| >40% | 20.04 | 0.30 (0.18, 0.51) | <0.0001 | |
| NYHA functional class | 3974/377 (91.3%) | |||
| II | 1.00 (reference) | |||
| III | 7.30 | 3.28 (1.39, 7.76) | 0.0069 | |
| IV | 20.26 | 6.72 (2.93, 15.4) | <0.0001 | |
| Ischemic etiology | 4346/5 (99.9%) | 6.14 | 1.49 (1.09, 2.08) | 0.0132 |
| Prior admission for heart failure | 4315/36 (99.2%) | 25.24 | 2.21 (1.62, 3.01) | <0.0001 |
| Diabetes mellitus | 4345/6 (99.9%) | 3.38 | 1.34 (0.98, 1.83) | 0.0658 |
| Atrial fibrillation | 4337/14 (99.7%) | 0.76 | 0.87 (0.63, 1.19) | 0.3841 |
| Stroke | 4334/17 (99.6%) | 2.83 | 1.39 (0.95, 2.03) | 0.0925 |
| COPD | 4329/22 (99.5%) | 6.37 | 1.99 (1.17, 3.39) | 0.0116 |
| Hemoglobin (per 1 mg/dL increase) | 4345/6 (99.9%) | 39.52 | 0.81 (0.75, 0.86) | <0.0001 |
| GFR (per 1 mL/min/1.73 m2 increase) | 4323/28 (99.4%) | 38.40 | 0.98 (0.97, 0.98) | <0.0001 |
| BUN (per 1 mg/dL increase) | 4343/8 (99.9%) | 118.25 | 1.03 (1.03, 1.04) | <0.0001 |
| Total bilirubin (per 1 mg/dL increase) | 4205/146 (96.6%) | 17.40 | 1.35 (1.17, 1.55) | <0.0001 |
| Albumin (per 1 mg/dL increase) | 2834/1317 (65.1%) | 95.06 | 0.22 (0.17, 0.30) | <0.0001 |
| Hyponatremia (sodium ≤ 135 mEq/L) | 4344/7 (99.9%) | 45.62 | 3.23 (2.33, 4.55) | <0.0001 |
| BNP >1000 pg/mLor NT-proBNP >4000 pg/mL | 4219/132 (97.0%) | 58.46 | 4.05 (2.83, 5.80) | <0.0001 |
| Loop diuretics at baseline | 4204/147 (96.6%) | 18.21 | 2.01 (1.46, 2.76) | <0.0001 |
| ACEI or ARB at baseline | 4318/33 (99.2%) | 4.37 | 0.71 (0.52, 0.98) | 0.0366 |
| Beta blockers at baseline | 4313/38 (99.1%) | 0.29 | 0.92 (0.67, 1.26) | 0.5929 |
| Aldosterone antagonists at baseline | 4177/174 (96.0%) | 5.86 | 1.58 (1.09, 2.28) | 0.0155 |
| Variable | Beta Coefficient | OR | 95% CI | p Value |
|---|---|---|---|---|
| Age | ||||
| <85 y/o | 1.00 | Reference | ||
| ≥85 y/o | 0.772 | 2.16 | (1.54, 3.04) | <0.0001 |
| SBP | ||||
| >140 mm Hg | 1.00 | Reference | ||
| 100−140 mm Hg | 0.496 | 1.64 | (1.13, 2.39) | 0.009 |
| <100 mm Hg | 1.386 | 4.00 | (2.51, 6.36) | <0.0001 |
| BUN | ||||
| <40 mg/dL | 1.00 | Reference | ||
| ≥40 mg/dL | 0.889 | 2.43 | (1.73, 3.43) | <0.0001 |
| Sodium | ||||
| >135 mEq/L | 1.00 | Reference | ||
| ≤135 mEq/L | 0.755 | 2.13 | (1.46, 3.10) | <0.0001 |
| Albumin | ||||
| >3.0 mg/dL | 1.00 | Reference | ||
| ≤3.0 mg/dL | 1.428 | 4.17 | (2.82, 6.16) | <0.0001 |
| Natriuretic peptides | ||||
| BNP <1000 or NT-proBNP <4000 pg/mL | 1.00 | Reference | ||
| BNP ≥1000 or NT-proBNP ≥4000 pg/mL | 1.063 | 2.90 | (1.97, 4.25) | <0.0001 |
| Variable | Risk Score | ||||
|---|---|---|---|---|---|
| Age (years) | <85 | ≥85 | |||
| 0 | +2 | ||||
| SBP (mmHg) | >140 | 100–140 | <100 | ||
| 0 | +1 | +3 | |||
| BUN (mg/dL) | <40 | ≥40 | |||
| 0 | +2 | ||||
| Sodium (mEq/L) | >135 | ≤135 | Total score | Mortality rate | |
| 0 | +2 | ||||
| Albumin (mg/dL) | >3.0 | ≤3.0 | 0–1 2–4 5–6 7–8 9–14 | <1% 1–5% 5–10% 10–30% >30% | |
| 0 | +3 | ||||
| BNP or NT-proBNP (pg/mL) | <1000 or 4000 | ≥1000 or 4000 | |||
| 0 | +2 | ||||
| Total score = | |||||
| Variable | Low (Score 0–1) | Intermediate (Score 2–4) | High (Score 5–14) |
|---|---|---|---|
| Loop diuretics, IV, % | 64.4 | 69.4 | 74.0 * |
| Vasodilators, IV, % | 60.2 | 66.5 | 57.8 * |
| Inotropes, IV, % | 6.9 | 14.9 | 27.7 * |
| Non-invasive ventilation, % | 16.3 | 22.7 | 21.4 * |
| Intubation, % | 3.1 | 4.7 | 4.8 |
| IABP, % | 0.8 | 2.3 | 3.1 * |
| VA-ECMO, % | 0.3 | 0.6 | 0.6 |
| Dialysis, % | 0.6 | 4.6 | 5.8 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiraishi, Y.; Kohsaka, S.; Abe, T.; Nagai, T.; Goda, A.; Nishihata, Y.; Nagatomo, Y.; Saji, M.; Toyosaki, Y.; Takei, M.; et al. Derivation and Validation of Clinical Prediction Models for Rapid Risk Stratification for Time-Sensitive Management for Acute Heart Failure. J. Clin. Med. 2020, 9, 3394. https://doi.org/10.3390/jcm9113394
Shiraishi Y, Kohsaka S, Abe T, Nagai T, Goda A, Nishihata Y, Nagatomo Y, Saji M, Toyosaki Y, Takei M, et al. Derivation and Validation of Clinical Prediction Models for Rapid Risk Stratification for Time-Sensitive Management for Acute Heart Failure. Journal of Clinical Medicine. 2020; 9(11):3394. https://doi.org/10.3390/jcm9113394
Chicago/Turabian StyleShiraishi, Yasuyuki, Shun Kohsaka, Takayuki Abe, Toshiyuki Nagai, Ayumi Goda, Yosuke Nishihata, Yuji Nagatomo, Mike Saji, Yuichi Toyosaki, Makoto Takei, and et al. 2020. "Derivation and Validation of Clinical Prediction Models for Rapid Risk Stratification for Time-Sensitive Management for Acute Heart Failure" Journal of Clinical Medicine 9, no. 11: 3394. https://doi.org/10.3390/jcm9113394






