The Association between Prostate-Specific Antigen Velocity (PSAV), Value and Acceleration, and of the Free PSA/Total PSA Index or Ratio, with Prostate Conditions
Abstract
:1. Introduction
2. Methods
2.1. Design
- (1)
- PSA greater than 4 ng/mL and free PSAl/total PSA index less than 15%;
- (2)
- Suspicious rectal examination;
- (3)
- Positive PSA velocity higher than 0.75 ng/mL in 12 months.
2.2. Groups by Serum PSA Level
- -
- The value of PSA in all of the 2035 individuals: mean 2.01, SD 10.01, median 1.1, range 0.04–435.94;
- -
- The distribution of the values: on one hand, it was tried that the groups had a number of individuals as similar as possible. On the other hand, that the value of the PSA figures could have a clinical significance.
2.3. Variables
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
PSA Index (iPSA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BHP | Benign prostatic hyperplasia |
| CE | European Conformity (Conformité Européenne) |
| DRE | Digital rectal examination |
| FDA | U.S. Food and Drug Administration |
| GA | Group A |
| GB | Group B |
| GC | Group C |
| GRUMUR | Renal Urological Multidisciplinary Research Group |
| PCa | Prostate cancer |
| ProPSA | Precursor of PSA |
| PSA | Prostate-specific antigen |
| PSAV | Prostate-specific antigen velocity |
| PSA-DT | PSA duplication time |
| TRUS | Transrectal ultrasound |
References
- Padilla-Fernández, B.-Y. Tumor de Próstata. In Nefrourología, 1st ed.; Lorenzo-Gómez, M.-F., Macias-Nuñez, J.-F., Eds.; Cervantes Internacional: Cervantes, Salamanca, Spain, 2013; Volume 1, pp. 637–655. [Google Scholar]
- Salvatierra-Pérez, C.; Gil-Vicente, A.; Lorenzo-Gómez, M. Hiperplasia benigna de Próstata. In Nefrourología, © MF Lorenzo Gómez ed.; Lorenzo-Gomez, M.-F., Macías-Núñez, J.-F., Eds.; Cervantes Internacional: Cervantes, Salamanca, Spain, 2013; Volume II - Sección Urología; pp. 757–787. [Google Scholar]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamey, T.; Yang, N.; Hay, A.; McNeal, J.; Freiha, F.; Redwine, E. Prostate specific antigen as aserum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; deKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef]
- Semjonow, A.; Brandt, B.; Oberpenning, F.; Roth, S.; Hertle, L. Discordance of assay methods creates pitfalls for the interpretation of prostate-specific antigen values. Prostate 1996, 29, 3–16. [Google Scholar] [CrossRef]
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D.; et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <or =4.0 ng per milliliter. N. Engl. J. Med. 2004, 350, 2239–2246. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Koyanagi, Y.; Inoue, T.; Fukuyama, T. [Some physico-chemical characteristics of “-seminoprotein”, an antigenic component specific for human seminal plasma. Forensic immunological study of body fluids and secretion. VII]. Nihon Hoigaku zasshi Jpn J. Leg. Med. 1971, 25, 322–324. [Google Scholar]
- Parkin, D.M.; Bray, F.I.; Devesa, S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 2001, 37 (Suppl. 8), 4–66. [Google Scholar] [CrossRef]
- Carlson, G.; Calvanese, C.; Partin, A. An algorithm combining age, total prostate-specific antigen (PSA), and percent free PSA to predict prostate cancer: Results on 4298 cases. Urology 1998, 52, 455. [Google Scholar] [CrossRef]
- Stephan, C.; Lein, M.; Jung, K.; Schnorr, D.; Loening, S.A. The influence of prostate volume on the ratio of free to total prostate specific antigen in serum of patients with prostate carcinoma and benign prostate hyperplasia. Cancer 1997, 79, 104–109. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Slawin, K.M.; Brawer, M.K.; Flanigan, R.C.; Patel, A.; Richie, J.P.; deKernion, J.B.; Walsh, P.C.; Scardino, P.T.; et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: A prospective multicenter clinical trial. JAMA 1998, 279, 1542–1547. [Google Scholar] [CrossRef]
- Catalona, W.; Bartsch, G.; Rittenhouse, H.; Evans, C.; Linton, H.; Amirkhan, A. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J. Urol. 2003, 170, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Sokoll, L.; Chan, D.; Mikolajczyk, S.; Rittenhouse, H.; Evans, C.; Linton, H. Proenzyme psa for the early detection of prostate cancer in the 2.5-4.0 ng/ml total psa range: Preliminary analysis. Urology 2003, 61, 274–276. [Google Scholar] [CrossRef]
- Carter, H.B.; Pearson, J.D.; Metter, E.J.; Brant, L.J.; Chan, D.W.; Andres, R.; Fozard, J.L.; Walsh, P.C. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA 1992, 267, 2215–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, H.P.; McNeal, J.E.; Stamey, T.A. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer 1993, 71, 2031–2040. [Google Scholar] [CrossRef]
- Arlen, P.M.; Bianco, F.; Dahut, W.L.; D’Amico, A.; Figg, W.D.; Freedland, S.J.; Gulley, J.L.; Kantoff, P.W.; Kattan, M.W.; Lee, A.; et al. Re: Prostate specific antigen working group guidelines on prostate specific antigen doubling time. J. Urol. 2008, 179, 2181–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, H.B.; Morrell, C.H.; Pearson, J.D.; Brant, L.J.; Plato, C.C.; Metter, E.J.; Chan, D.W.; Fozard, J.L.; Walsh, P.C. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 1992, 52, 3323–3328. [Google Scholar]
- Carter, H.B.; Coffey, D.S. The prostate: An increasing medical problem. Prostate 1990, 16, 39–48. [Google Scholar] [CrossRef]
- Lorenzo-Gómez, M.-F. Sujetos participantes en la investigación. Garantías. In Guía de Buenas Prácticas en Investigación; Comisión-de-Investigación-del-Complejo-Asistencial-Universitario-de-Salamanca©, Ed.; Comisión de Investigación del Complejo Asistencial Universitario de Salamanca©: Salamanca, Spain, 2015; Volume 1, pp. 41–53. [Google Scholar]
- Barry, M.J.; Fowler, F.J., Jr.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Loughlin, K.R. PSA velocity: A systematic review of clinical applications. Urologic Oncol. 2014, 32, 1116–1125. [Google Scholar] [CrossRef]
- San-Bartolomé-Gutiérrez, J. Estudio de Incidencia de Cáncer de Próstata en la Provincia de Salamanca; Original Research; Universidad de Salamanca (España): Salamanca, Spain, 2017. [Google Scholar]
- Vickers, A.J.; Thompson, I.M.; Klein, E.; Carroll, P.R.; Scardino, P.T. A commentary on PSA velocity and doubling time for clinical decisions in prostate cancer. Urology 2014, 83, 592–596. [Google Scholar] [CrossRef]
- Taha, A.S.; Hudson, N.; Hawkey, C.J.; Swannell, A.J.; Trye, P.N.; Cottrell, J.; Mann, S.G.; Simon, T.J.; Sturrock, R.D.; Russell, R.I. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1996, 334, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López-Ortiz, C.-G.; Hernández-Bueno, J.-A.; González-Bárcena, D.; López-Gamboa, M.; Ortiz-Plata, A.; Porias-Cuéllar, H.-L.; Rembao-Bojórquez, J.-D.; Sandoval-Huerta, G.-A.; Tapia-Serrano, R.; Vazquez-Castillo, G.-G.; et al. Guia de práctica clínica para el diagnóstico y tratamiento de la hiperprolactinemia. Ginecol. Obstet. Mex. 2014, 82, 123–142. [Google Scholar] [PubMed]
- Ruiz López, A.I.; Pérez Mesa, J.C.; Borrego Chi, Y.; Cruz Batista, Y. Testosterona y antígeno prostático específico en pacientes portadores de carcinoma prostático, provincia Holguín, 2013–2015. Correo Cient. Méd. 2017, 21, 720–733. [Google Scholar]
- Rivera, P.; Tagle, R.; Mir, S.; González, R. Relación entre niveles de tertosterona en suero y cáncer prostático. Actas Urol. Esp. 2003, 27, 788–792. [Google Scholar] [CrossRef]

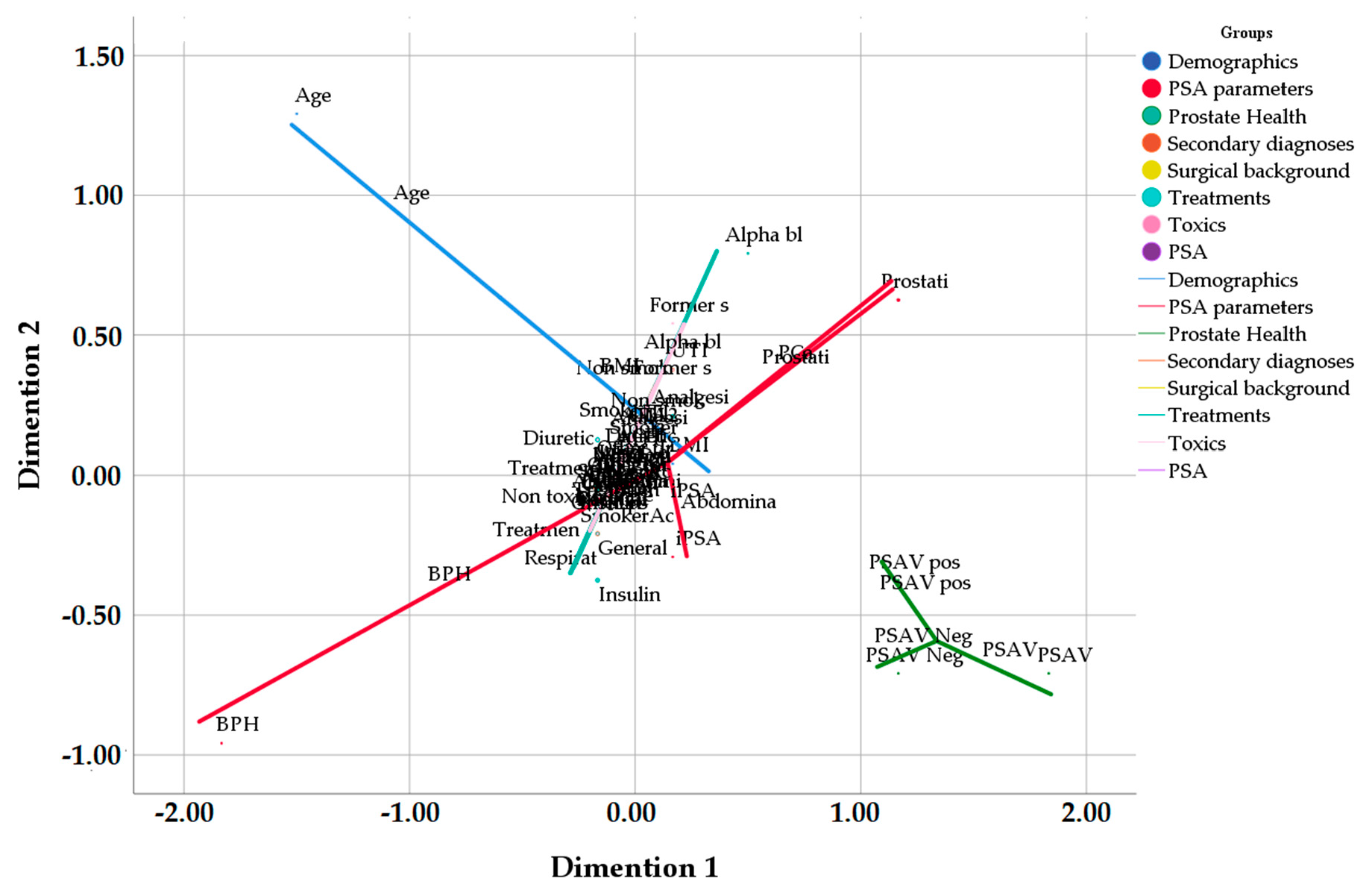
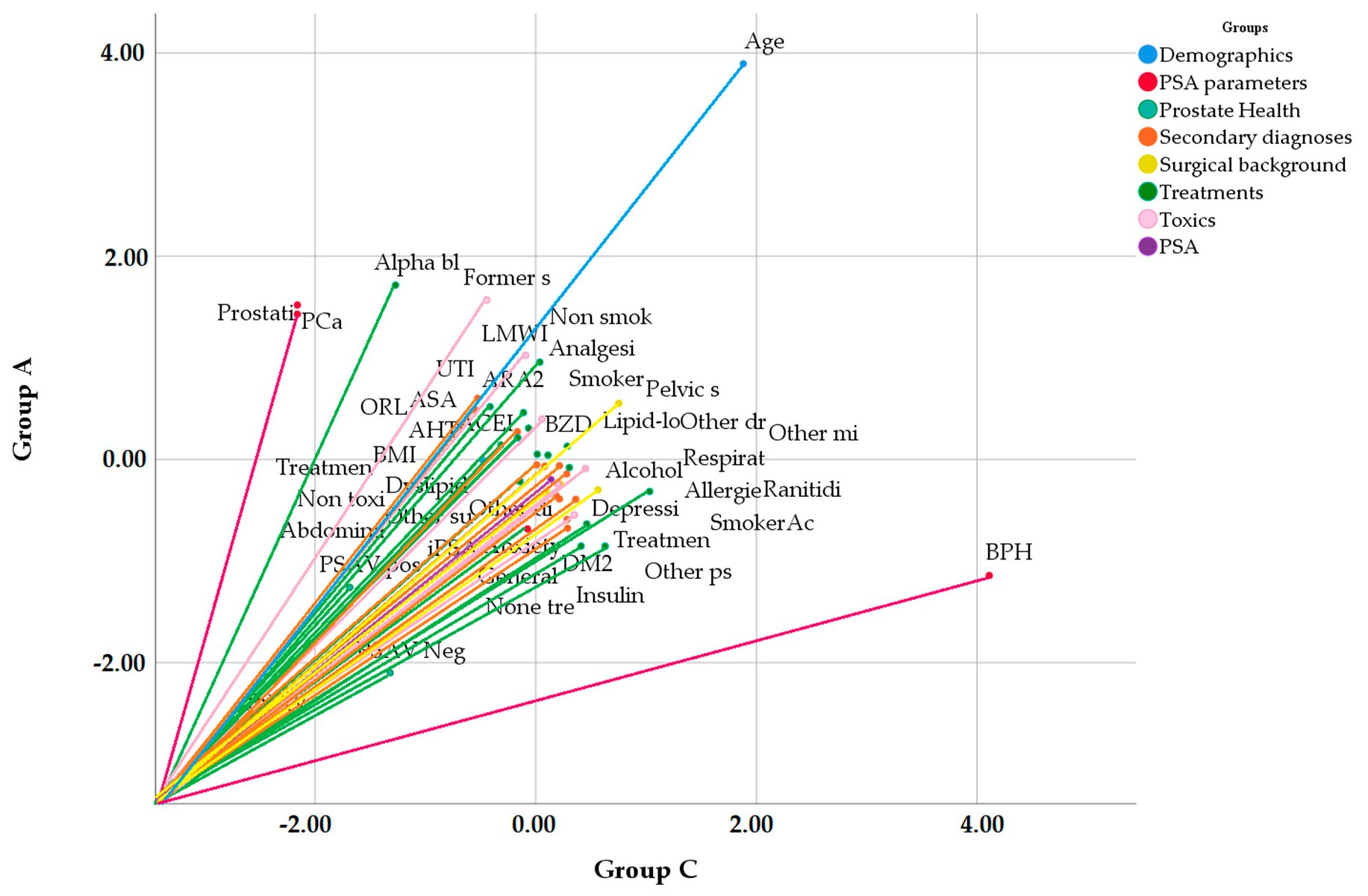
| Variable | Group | p | |||||
|---|---|---|---|---|---|---|---|
| GA | GB | GC | |||||
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | ||
| Age | 65.80 (8.24) | 65 (50–94) | 62.24 (7.68) | 62 (47–93) | 60.07 (7.66) | 58 (49–94) | 0.0001 |
| BMI | 27.49 (3.61) | 27.18 (19.75–46.25) | 27.93 (4.19) | 27.68 (18.56–57.13) | 28.06 (3.90) | 27.68 (19.41–43.52) | 0.0755 |
| PSAV | 1.56 (3.53) | 0.36 (0.0013–34.46) | 0.37 (1.02) | 0.11 (0.001–17.88) | 0.36 (1.36) | 0.057 (0.001–20.96) | 0.0001 |
| iPSA | 20.65 (9.46) | 18.68 (3.7–75.85) | 26 (11.93) | 23.55( 6.02–75.78) | 33.57 (16.51) | 29.63 (6.25–115) | 0.0001 |
| PSAV acceleration | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | 0.00001 |
| 305/69.16 | 136/30.84 | 355/57.54 | 262/42.46 | 239/41.28 | 340/58.72 | ||
| PRIMARY DIAGNOSIS | n | % | n | % | n | % | p |
| PD: BPH | 258 | 48.81 | 664 | 86.68 | 678 | 91.32 | 0.00001 |
| PD: PIN—non-infectious prostatitis | 158 | 30.50 | 106 | 13.68 | 58 | 7.82 | |
| PD: PCa | 102 | 19.69 | 5 | 0.65 | 6 | 0.81 | |
| Gleason | 7.28 (0.80) | 7.00 (6–8) | 6.53 (0.50) | 7.00 (6–7) | 4.62 (0.48) | 5.00 (4–5) | 0.006 |
| Variable | Group | p | |||||
|---|---|---|---|---|---|---|---|
| GAP Ca+ | GB PCa+ | GC PCa+ | |||||
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | ||
| Age | 67.59 (8.16) | 66.50 (53.00–86.00) | 62.40 (7.76) | 65.00 (55.00–72.00) | 66.33 (7.20) | 68.00 (53.00–74.00) | 1.0000 |
| BMI | 26.85 (3.38) | 26.00 (20.00–36.00) | 29.30 (2.00) | 29.00 (27.00–31.00) | 27.33 (3.50) | 26.50 (23.00–32.00) | 0.762 |
| PSAV | 3.21 (5.49) | 1.38 (0.001–34.46) | 0.20 (0.17) | 0.19 (0.07–0.32)) | 2.25 (0.20) | 2.20 (2.20–2.35) | 0.828 |
| iPSA | 16.40 (8.39) | 14.76 (5.03–48.53) | 21.55 (13.87) | 16.35 (7.06–39.29) | 26.41 (6.67) | 26.39 (18.20–34.94) | 0.0041 |
| PSAV acceleration | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | 0.0036 |
| 76/74.5 | 26/25.50 | 2/30.00 | 3/70.00 | 1/16.70 | 5/83.30 | ||
| Gleason | 7.47 (0.50) | 7.00 (7–8) | 6.60 (0.54) | 7.00 (6–7) | 5.10 (0.10) | 5.00 (5–5.10) | 0.00004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Fraile, M.-C.; Padilla-Fernández, B.Y.; Valverde-Martínez, S.; Marquez-Sanchez, M.; García-Cenador, M.-B.; Lorenzo-Gómez, M.-F.; Flores-Fraile, J. The Association between Prostate-Specific Antigen Velocity (PSAV), Value and Acceleration, and of the Free PSA/Total PSA Index or Ratio, with Prostate Conditions. J. Clin. Med. 2020, 9, 3400. https://doi.org/10.3390/jcm9113400
Flores-Fraile M-C, Padilla-Fernández BY, Valverde-Martínez S, Marquez-Sanchez M, García-Cenador M-B, Lorenzo-Gómez M-F, Flores-Fraile J. The Association between Prostate-Specific Antigen Velocity (PSAV), Value and Acceleration, and of the Free PSA/Total PSA Index or Ratio, with Prostate Conditions. Journal of Clinical Medicine. 2020; 9(11):3400. https://doi.org/10.3390/jcm9113400
Chicago/Turabian StyleFlores-Fraile, María-Carmen, Bárbara Yolanda Padilla-Fernández, Sebastián Valverde-Martínez, Magaly Marquez-Sanchez, María-Begoña García-Cenador, María-Fernanda Lorenzo-Gómez, and Javier Flores-Fraile. 2020. "The Association between Prostate-Specific Antigen Velocity (PSAV), Value and Acceleration, and of the Free PSA/Total PSA Index or Ratio, with Prostate Conditions" Journal of Clinical Medicine 9, no. 11: 3400. https://doi.org/10.3390/jcm9113400
APA StyleFlores-Fraile, M.-C., Padilla-Fernández, B. Y., Valverde-Martínez, S., Marquez-Sanchez, M., García-Cenador, M.-B., Lorenzo-Gómez, M.-F., & Flores-Fraile, J. (2020). The Association between Prostate-Specific Antigen Velocity (PSAV), Value and Acceleration, and of the Free PSA/Total PSA Index or Ratio, with Prostate Conditions. Journal of Clinical Medicine, 9(11), 3400. https://doi.org/10.3390/jcm9113400







