In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phantom Studies
2.2. Study Population
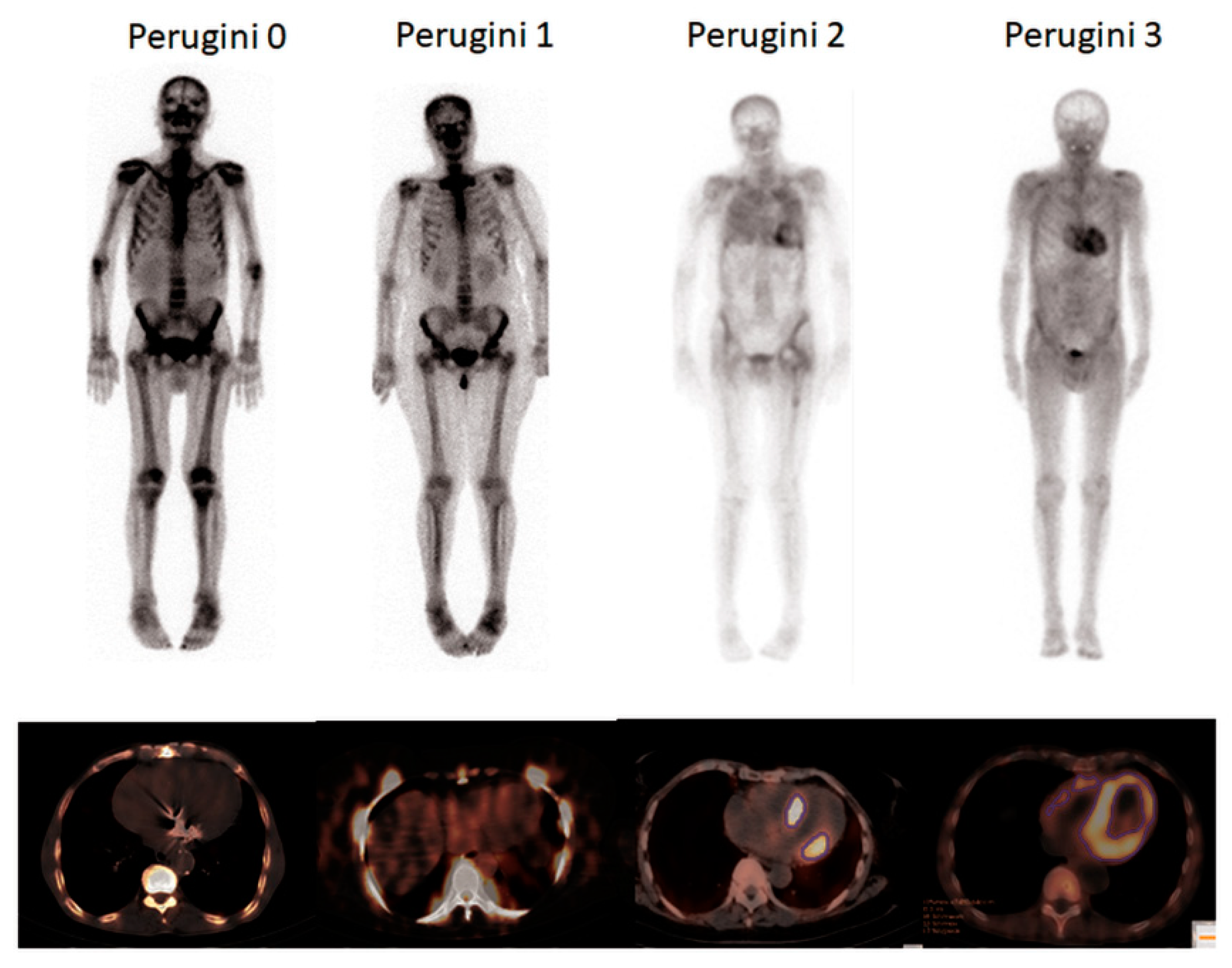
2.3. Bone Scan
2.4. Image Analysis
2.5. Genetic Analyses
2.6. CMR
2.7. Statistical Analysis
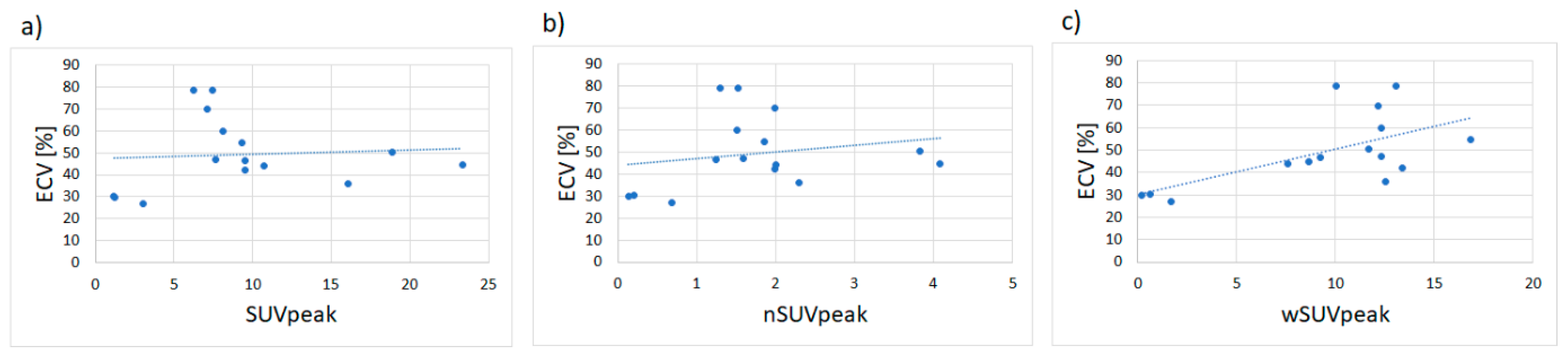
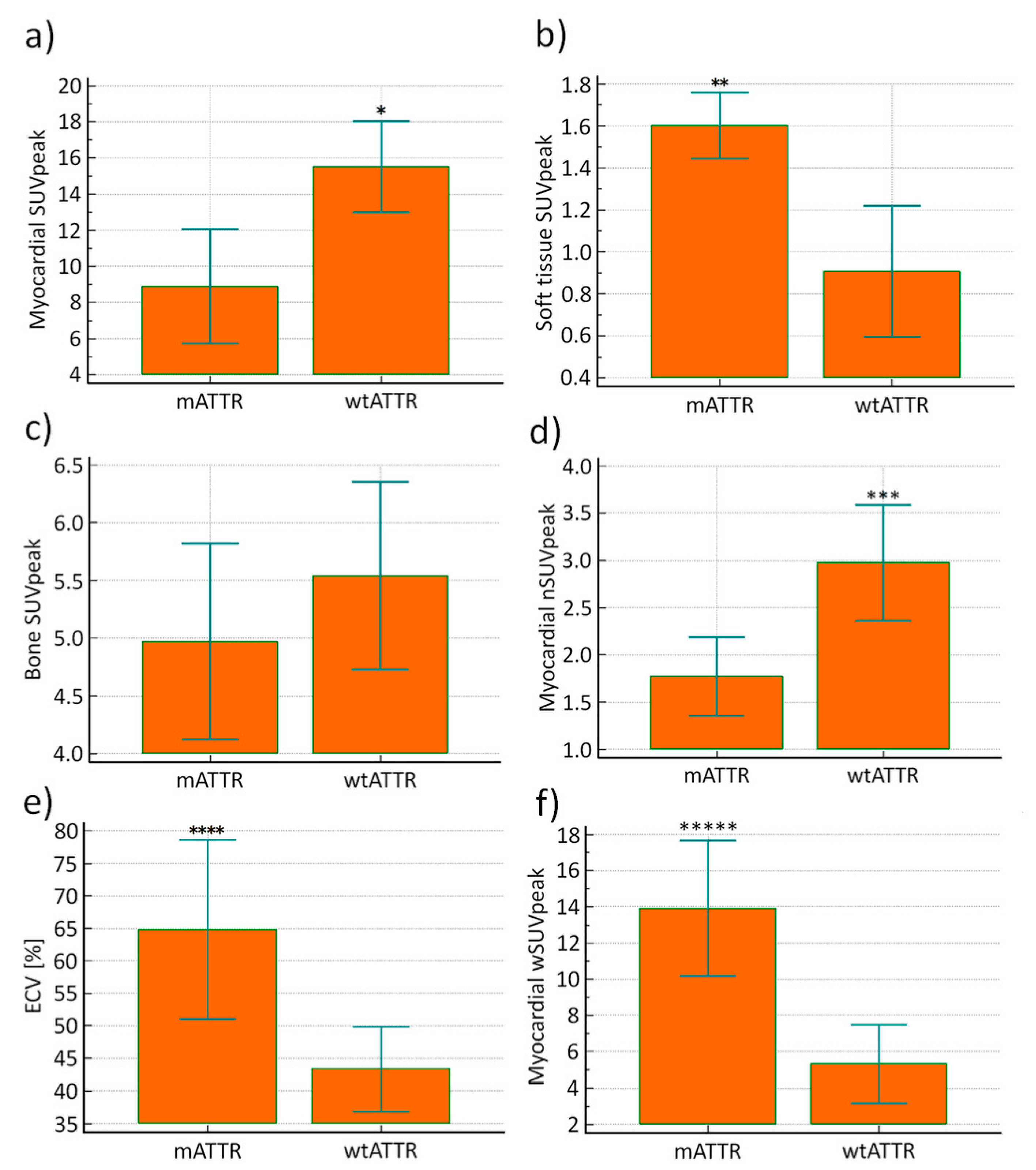
3. Results
3.1. Phantom Experiments
3.2. Study Population
3.3. Bone Scans
3.4. Genetic Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lachmann, H.J.; Hawkins, P.N. Systemic amyloidosis. Curr. Opin. Pharmacol. 2006, 6, 214–220. [Google Scholar] [CrossRef]
- McCarthy, R.E., 3rd; Kasper, E.K. A review of the amyloidoses that infiltrate the heart. Clin. Cardiol. 1998, 21, 547–552. [Google Scholar] [CrossRef]
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and treatment of cardiac amyloidosis. Nat. Rev. Cardiol. 2014, 12, 91. [Google Scholar] [CrossRef]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.J.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Berk, J.L.; Suhr, O.B.; Obici, L.; Sekijima, Y.; Zeldenrust, S.R.; Yamashita, T.; Heneghan, M.A.; Gorevic, P.D.; Litchy, W.J.; Wiesman, J.F.; et al. Repurposing Diflunisal for Familial Amyloid Polyneuropathy: A Randomized Clinical Trial. JAMA 2013, 310, 2658–2667. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; Adams, D.; Kristen, A.; Grogan, M.; González-Duarte, A.; Maurer, M.S.; Merlini, G.; Damy, T.; Slama, M.S.; Brannagan, T.H.; et al. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients With Hereditary Transthyretin-Mediated Amyloidosis. Circulation 2019, 139, 431–443. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Sperry, B.W.; Vranian, M.N.; Hachamovitch, R.; Joshi, H.; McCarthy, M.; Ikram, A.; Hanna, M. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int. J. Cardiol. 2016, 214, 477–481. [Google Scholar] [CrossRef]
- Falk, R.H.; Quarta, C.C. Echocardiography in cardiac amyloidosis. Heart Fail. Rev. 2015, 20, 125–131. [Google Scholar] [CrossRef]
- Banypersad, S.M.; Moon, J.C.; Whelan, C.; Hawkins, P.N.; Wechalekar, A.D. Updates in cardiac amyloidosis: A review. J. Am. Heart Assoc. 2012, 1, e000364. [Google Scholar] [CrossRef] [Green Version]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442. [Google Scholar] [CrossRef]
- Vogelsberg, H.; Mahrholdt, H.; Deluigi, C.C.; Yilmaz, A.; Kispert, E.M.; Greulich, S.; Klingel, K.; Kandolf, R.; Sechtem, U. Cardiovascular Magnetic Resonance in Clinically Suspected Cardiac Amyloidosis: Noninvasive Imaging Compared to Endomyocardial Biopsy. J. Am. Coll. Cardiol. 2008, 51, 1022–1030. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Ribeiro, V.F.; de Oliveira, D.C.L.; das Neves, D.G.; Nunes, N.S.V.; Villacorta Junior, H.; Nacif, M.S. Cardiac Magnetic Resonance and amyloidosis: Review. Int. J. Cardiovasc. Sci. 2019, 32, 177–189. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Rutten, K.H.G.; Minnema, M.C.; Raymakers, R.A.P.; Asselbergs, F.W.; de Jonge, N. Cardiac amyloidosis: The need for early diagnosis. Neth. Heart J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Knight, D.S.; Zumbo, G.; Barcella, W.; Steeden, J.A.; Muthurangu, V.; Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Kotecha, T.; et al. Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles. JACC Cardiovasc. Imaging 2019, 12, 823–833. [Google Scholar] [CrossRef]
- Banypersad, S.M.; Sado, D.M.; Flett, A.S.; Gibbs, S.D.J.; Pinney, J.H.; Maestrini, V.; Cox, A.T.; Fontana, M.; Whelan, C.J.; Wechalekar, A.D.; et al. Quantification of Myocardial Extracellular Volume Fraction in Systemic AL Amyloidosis. Circ. Cardiovasc. Imaging 2013, 6, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Duca, F.; Kammerlander, A.A.; Panzenböck, A.; Binder, C.; Aschauer, S.; Loewe, C.; Agis, H.; Kain, R.; Hengstenberg, C.; Bonderman, D.; et al. Cardiac Magnetic Resonance T1 Mapping in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2018, 11, 1924–1926. [Google Scholar] [CrossRef]
- Willerson, J.T.; Parkey, R.W.; Bonte, F.J.; Lewis, S.E.; Corbett, J.; Buja, L.M. Pathophysiologic considerations and clinicopathological correlates of technetium-99m stannous pyrophosphate myocardial scintigraphy. Semin. Nucl. Med. 1980, 10, 54–69. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Caobelli, F.; Braun, M.; Haaf, P.; Wild, D.; Zellweger, M.J. Quantitative 99mTc-DPD SPECT/CT in patients with suspected ATTR cardiac amyloidosis: Feasibility and correlation with visual scores. J. Nucl. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Haq, M.; Narotsky, D.L.; Goldsmith, J.; Weinberg, R.L.; Morgenstern, R.; Pozniakoff, T.; Ruberg, F.L.; Miller, E.J.; Berk, J.L.; et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA Cardiol. 2016, 1, 880–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. 99mTc-Pyrophosphate Scintigraphy for Differentiating Light-Chain Cardiac Amyloidosis From the Transthyretin-Related Familial and Senile Cardiac Amyloidoses. Circ. Cardiovasc. Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Gallini, C.; Tutino, F.; Martone, R.; Ciaccio, A.; Costanzo, E.N.; Taborchi, G.; Morini, S.; Bartolini, S.; Farsetti, S.; Di Mario, C.; et al. Semi-quantitative indices of cardiac uptake in patients with suspected cardiac amyloidosis undergoing 99mTc-HMDP scintigraphy. J. Nucl. Cardiol. 2019. [Google Scholar] [CrossRef]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of 99mTc-DPD Scintigraphy in Diagnosis and Prognosis of Hereditary Transthyretin-Related Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, I.S.; Hoffmann, S.A. Activity concentration measurements using a conjugate gradient (Siemens xSPECT) reconstruction algorithm in SPECT/CT. Nucl. Med. Commun. 2016, 37, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Delcroix, O.; Robin, P.; Gouillou, M.; Le Duc-Pennec, A.; Alavi, Z.; Le Roux, P.-Y.; Abgral, R.; Salaun, P.-Y.; Bourhis, D.; Querellou, S. A new SPECT/CT reconstruction algorithm: Reliability and accuracy in clinical routine for non-oncologic bone diseases. EJNMMI Res. 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, S.C.; Lindsay, K.; Fong, W.; Patford, S.; Younger, J.; Atherton, J. Tc-HDP quantitative SPECT/CT in transthyretin cardiac amyloid and the development of a reference interval for myocardial uptake in the non-affected population. Eur. J. Hybrid Imaging 2018, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [Green Version]
- Vija, A.H. Introduction to xSPECT technology: Evolving multi-modal SPECT to become context-based and quantitative. Siemens Med Solut. USA Inc. Mol. Imaging White Pap. 2013. Available online: https://www.siemens-healthineers.com/pl/obrazowanie-molekularne/customer-portal-resource/expand-scanner-capabilities/symbia-intevo (accessed on 9 August 2020).
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.J.; Huang, S.-C.; Phelps, M.E. Quantitation in Positron Emission Computed Tomography: 1. Effect of Object Size. J. Comput. Assist. Tomogr. 1979, 3, 299–308. [Google Scholar] [CrossRef]
- Cachovan, M.; Vija, A.H.; Hornegger, J.; Kuwert, T. Quantification of 99mTc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Scully, P.R.; Morris, E.; Patel, K.P.; Treibel, T.A.; Burniston, M.; Klotz, E.; Newton, J.D.; Sabharwal, N.; Kelion, A.; Manisty, C.; et al. DPD Quantification in Cardiac Amyloidosis: A Novel Imaging Biomarker. JACC Cardiovasc. Imaging 2020, 13, 1353–1363. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Ross, J.C.; Hutt, D.F.; Burniston, M.; Page, J.; Steeden, J.A.; Gillmore, J.D.; Wechalekar, A.D.; Hawkins, P.N.; Fontana, M. Quantitation of 99mTc-DPD uptake in patients with transthyretin-related cardiac amyloidosis. Amyloid 2018, 25, 203–210. [Google Scholar] [CrossRef]
- Hutt, D.F.; Fontana, M.; Burniston, M.; Quigley, A.-M.; Petrie, A.; Ross, J.C.; Page, J.; Martinez-Naharro, A.; Wechalekar, A.D.; Lachmann, H.J.; et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1344–1350. [Google Scholar] [CrossRef]
- Antoni, G.; Lubberink, M.; Estrada, S.; Axelsson, J.; Carlson, K.; Lindsjö, L.; Kero, T.; Långström, B.; Granstam, S.-O.; Rosengren, S.; et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorbala, S.; Vangala, D.; Semer, J.; Strader, C.; Bruyere, J.R.; Di Carli, M.F.; Moore, S.C.; Falk, R.H. Imaging cardiac amyloidosis: A pilot study using 18F-florbetapir positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Ihne, S.; Brumberg, J.; Morbach, C.; Knop, S.; Kortüm, K.M.; Störk, S.; Buck, A.K.; Reiter, T.; Bauer, W.R.; et al. Detection of cardiac amyloidosis with (18)F-Florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Law, W.P.; Wang, W.; Moore, P.; Mollee, P.; Ng, A. Cardiac amyloid imaging with (18)F-florbetaben positron emission tomography: A pilot study. Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2017, 24, 162. [Google Scholar] [CrossRef]
- Dietemann, S.; Nkoulou, R. Amyloid PET imaging in cardiac amyloidosis: A pilot study using (18)F-flutemetamol positron emission tomography. Ann. Nucl. Med. 2019, 33, 624–628. [Google Scholar] [CrossRef]
- Osborne, D.R.; Acuff, S.N.; Stuckey, A.; Wall, J.S. A Routine PET/CT Protocol with Streamlined Calculations for Assessing Cardiac Amyloidosis Using (18)F-Florbetapir. Front. Cardiovasc. Med. 2015, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Quarta, C.C.; Gonzalez-Lopez, E.; Gilbertson, J.A.; Botcher, N.; Rowczenio, D.; Petrie, A.; Rezk, T.; Youngstein, T.; Mahmood, S.; Sachchithanantham, S.; et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur. Heart J. 2017, 38, 1905–1908. [Google Scholar] [CrossRef]


| Patient no. | Age | Gender | SUVpeak Myocardium | ECV [%] | Perguini Score | Suvpeak Bone | Genetic Mutation |
|---|---|---|---|---|---|---|---|
| 1 | 60 | F | 8.1 | 60 | 3 | 5.4 | His108Arg |
| 2 | 66 | M | 1.0 | - | 0 | 9.5 | |
| 3 | 88 | F | 10.7 | 43.9 | 2 | 5.4 | wtATTR |
| 4 | 79 | M | 2.3 | - | 1 | 8.0 | |
| 5 | 64 | M | 9.3 | 54.5 | 3 | 5.0 | Thr80Ala |
| 6 | 62 | F | 7.5 | 78.7 | 3 | 5.8 | His108Arg |
| 7 | 77 | M | 16.1 | 35.8 | 2 | 7 | wtATTR |
| 8 | 78 | F | 22.4 | - | 2 | 5.8 | wtATTR |
| 9 | 71 | F | 9.6 | 42.1 | 3 | 4.8 | wtATTR |
| 10 | 84 | M | 18.9 | 50.3 | 2 | 4.9 | wtATTR |
| 11 | 67 | M | 7.1 | 69.9 | 3 | 3.6 | His108Arg |
| 12 | 74 | M | 23.4 | 44.8 | 2 | 5.7 | wtATTR |
| 13 | 80 | M | 14.4 | - | 3 | 3.9 | wtATTR |
| 14 | 54 | F | 1.8 | - | 1 | 12.7 | |
| 15 | 87 | M | 1.2 | 30.1 | 1 | 5.8 | |
| 16 | 82 | M | 10.5 | - | 3 | 4.1 | wtATTR |
| 17 | 63 | F | 1.7 | - | 1 | 7.5 | |
| 18 | 78 | M | 8.4 | - | 3 | 7.6 | wtATTR |
| 19 | 86 | M | 9.5 | 46.6 | 2 | 7.6 | unknown |
| 20 | 79 | M | 18.8 | - | 3 | 3.6 | wtATTR |
| 21 | 48 | M | 6.2 | 78.8 | 3 | 4.1 | His108Arg |
| 22 | 70 | F | 16.4 | - | 3 | 6.2 | Val113Leu |
| 23 | 75 | M | 7.6 | 47 | 3 | 4.8 | Val50Met |
| 24 | 90 | M | 16.6 | - | 2 | 5.5 | wtATTR |
| 25 | 78 | M | 19.1 | - | 3 | 6.6 | wtATTR |
| 26 | 79 | M | 12.9 | - | 3 | 3.4 | wtATTR |
| 27 | 74 | M | 14.3 | - | 2 | 6.8 | wtATTR |
| 28 | 61 | F | 1.3 | - | 0 | 6.2 | |
| 29 | 79 | M | 1.9 | - | 0 | 7.3 | |
| 30 | 77 | M | 16.8 | - | 2 | 8.2 | wtATTR |
| 31 | 80 | M | 3.1 | 27 | 1 | 4.5 | |
| 32 | 45 | M | 1.2 | 29.76 | 0 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollenweber, T.; Rettl, R.; Kretschmer-Chott, E.; Rasul, S.; Kulterer, O.; Rainer, E.; Raidl, M.; Schaffarich, M.P.; Matschitsch, S.; Stadler, M.; et al. In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR). J. Clin. Med. 2020, 9, 3446. https://doi.org/10.3390/jcm9113446
Wollenweber T, Rettl R, Kretschmer-Chott E, Rasul S, Kulterer O, Rainer E, Raidl M, Schaffarich MP, Matschitsch S, Stadler M, et al. In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR). Journal of Clinical Medicine. 2020; 9(11):3446. https://doi.org/10.3390/jcm9113446
Chicago/Turabian StyleWollenweber, Tim, Rene Rettl, Elisabeth Kretschmer-Chott, Sazan Rasul, Oana Kulterer, Eva Rainer, Markus Raidl, Michael P. Schaffarich, Sabrina Matschitsch, Michael Stadler, and et al. 2020. "In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR)" Journal of Clinical Medicine 9, no. 11: 3446. https://doi.org/10.3390/jcm9113446
APA StyleWollenweber, T., Rettl, R., Kretschmer-Chott, E., Rasul, S., Kulterer, O., Rainer, E., Raidl, M., Schaffarich, M. P., Matschitsch, S., Stadler, M., Traub-Weidinger, T., Beiztke, D., Loewe, C., Duca, F., Mascherbauer, J., Bonderman, D., & Hacker, M. (2020). In Vivo Quantification of Myocardial Amyloid Deposits in Patients with Suspected Transthyretin-Related Amyloidosis (ATTR). Journal of Clinical Medicine, 9(11), 3446. https://doi.org/10.3390/jcm9113446






