Diagnosis and Management of Acute Coronary Syndrome: What is New and Why? Insight From the 2020 European Society of Cardiology Guidelines
Abstract
:1. Introduction
2. Diagnosis of Acute Coronary Syndrome
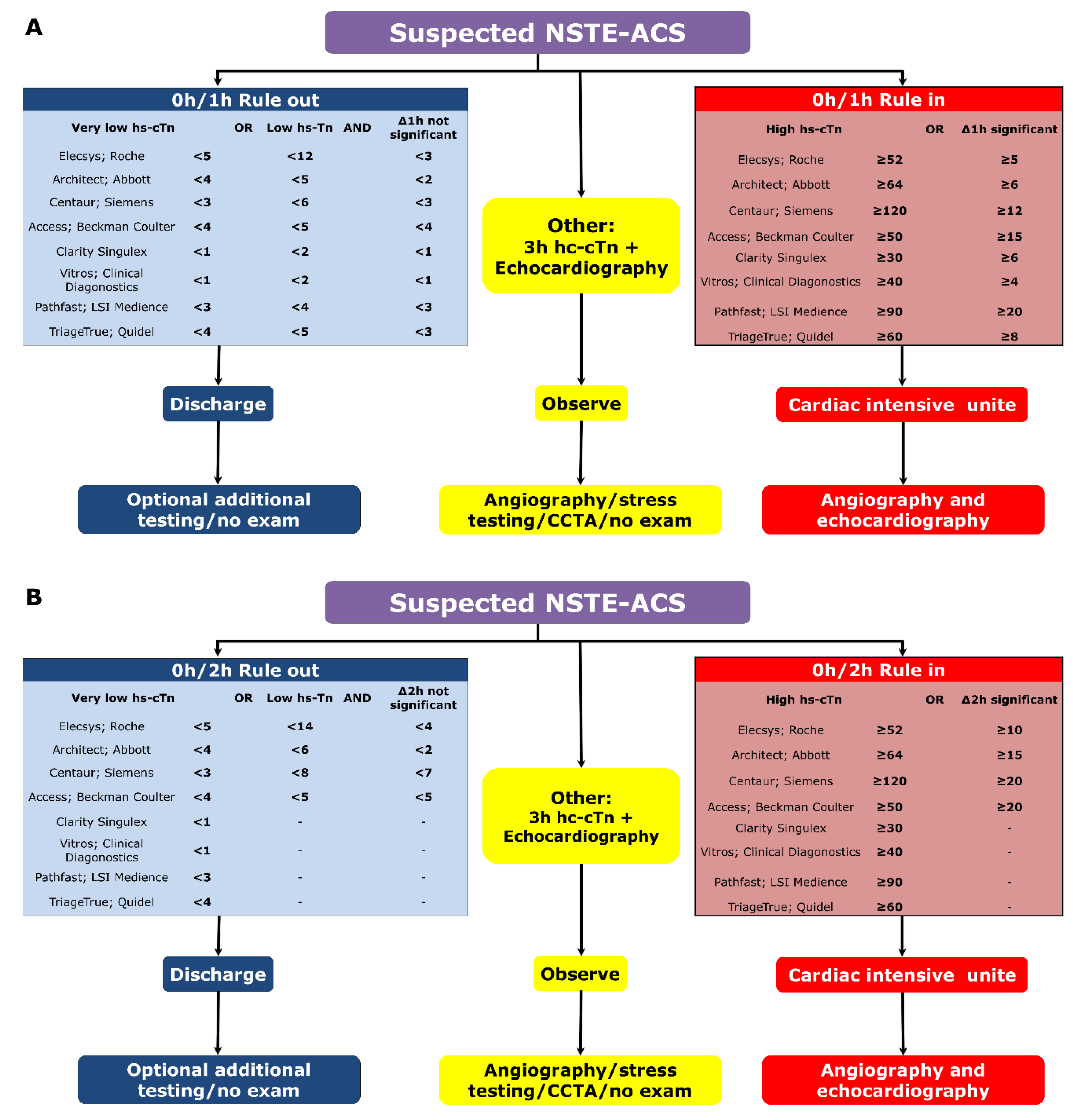
2.1. Rule-In, Rule-Out Algorithms
2.2. Other Biomarkers
3. Management of The Antithrombotic Treatment
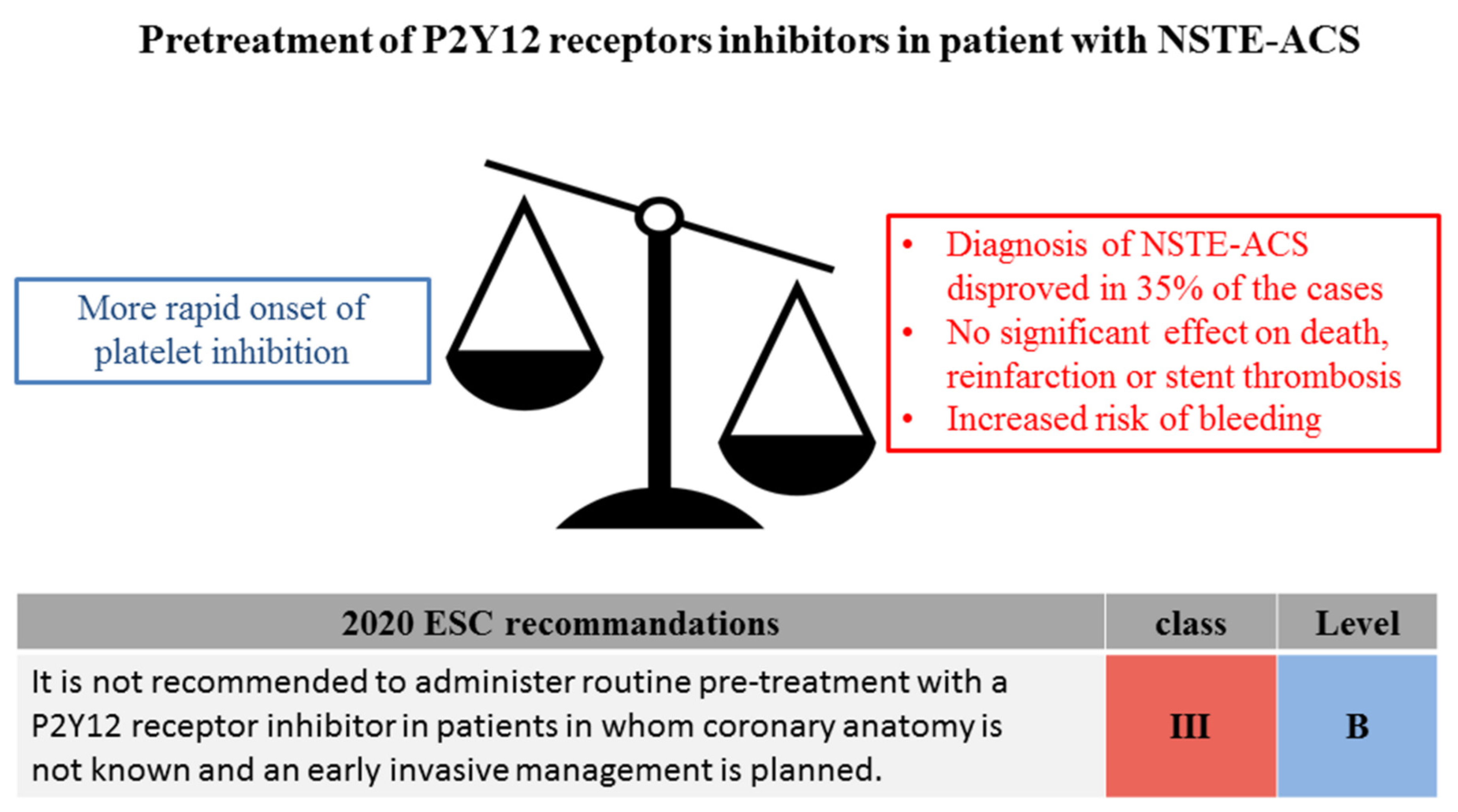
3.1. The Issue of Pretreatment
3.2. What P2Y12 Receptors Inhibitors Should Be Used?
3.3. What Antithrombotic Regimen Following PCI?
3.4. Risk Stratification
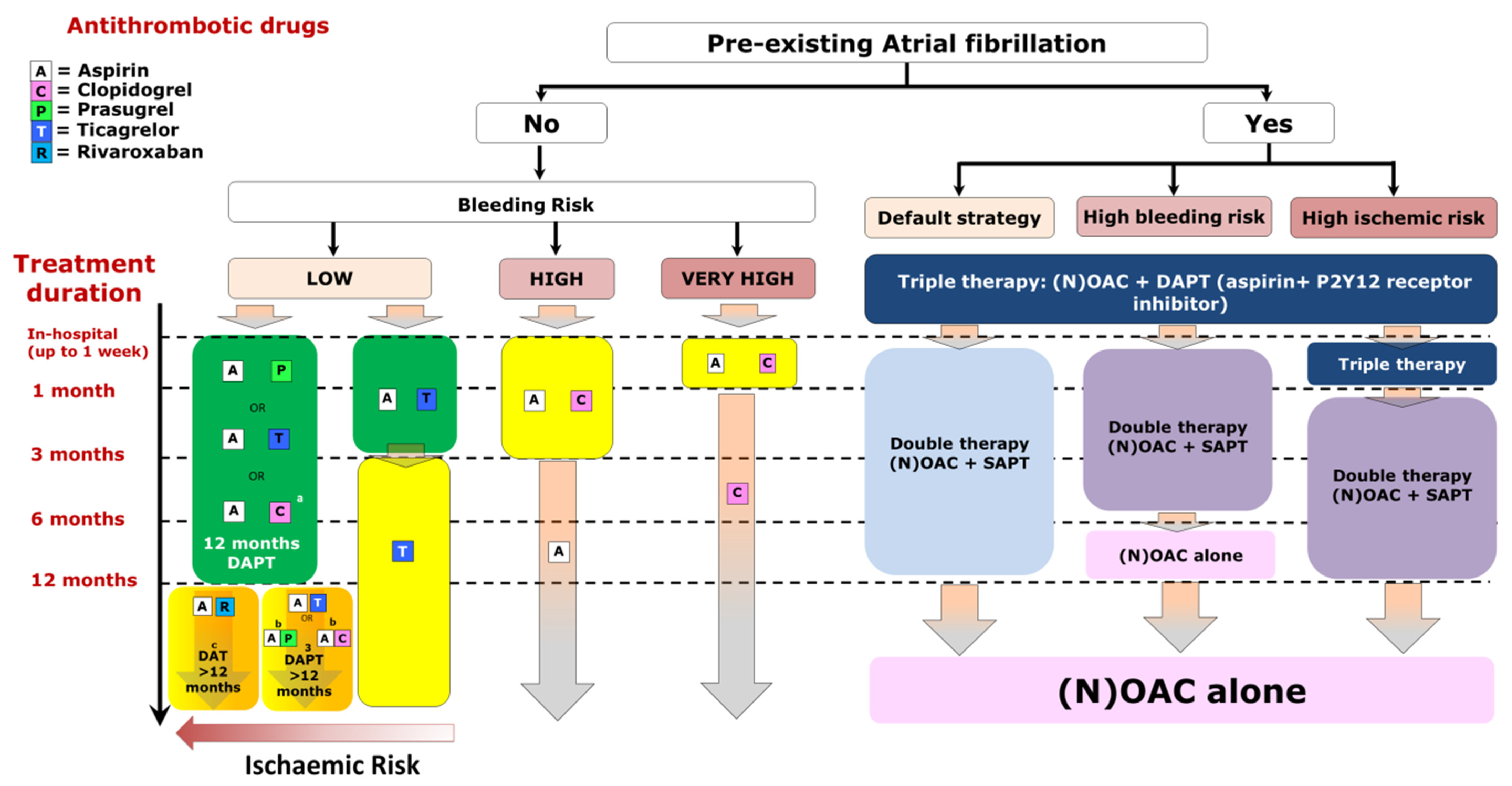
3.5. Pairing Chronic Oral Anticoagulation with Antiplatelet Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Aboyans, V.; Dalon, F.; Oksen, D.; Belhassen, M.; Nolin, M.; Briere, J.; van Ganse, E.; Montalescot, G. Epidemiology, treatment patterns and outcomes in patients with coronary or lower extremity artery disease in France. Arch. Cardiovasc. Dis. 2019, 112, 670–679. [Google Scholar] [CrossRef]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 1–79. [Google Scholar] [CrossRef]
- Nestelberger, T.; Boeddinghaus, J.; Greenslade, J.; Parsonage, W.A.; Than, M.; Wussler, D.; Lopez-Ayala, P.; Zimmermann, T.; Meier, M.; Troester, V.; et al. Two-Hour Algorithm for Rapid Triage of Suspected Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay. Clin. Chem. 2019, 65, 1437–1447. [Google Scholar] [CrossRef]
- Boeddinghaus, J.; Reichlin, T.; Cullen, L.; Greenslade, J.H.; Parsonage, W.A.; Hammett, C.; Pickering, J.W.; Hawkins, T.; Aldous, S.; Twerenbold, R.; et al. Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin. Chem. 2016, 62, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Reichlin, T.; Cullen, L.; Parsonage, W.A.; Greenslade, J.; Twerenbold, R.; Moehring, B.; Wildi, K.; Mueller, S.; Zellweger, C.; Mosimann, T.; et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am. J. Med. 2015, 128, 369–379. [Google Scholar] [CrossRef]
- Badertscher, P.; Boeddinghaus, J.; Twerenbold, R.; Nestelberger, T.; Wildi, K.; Wussler, D.; Schwarz, J.; Puelacher, C.; Rubini, G.M.; Kozhuharov, N.; et al. Direct Comparison of the 0/1h and 0/3h Algorithms for Early Rule-Out of Acute Myocardial Infarction. Circulation 2018, 137, 2536–2538. [Google Scholar] [CrossRef]
- Chapman, A.R.; Anand, A.; Boeddinghaus, J.; Ferry, A.V.; Sandeman, D.; Adamson, P.D.; Andrews, J.; Tan, S.; Cheng, S.F.; D’Souza, M.; et al. Comparison of the Efficacy and Safety of Early Rule-Out Pathways for Acute Myocardial Infarction. Circulation 2017, 135, 1586–1596. [Google Scholar] [CrossRef]
- Chapman, A.R.; Fujisawa, T.; Lee, K.K.; Andrews, J.P.; Anand, A.; Sandeman, D.; Ferry, A.V.; Stewart, S.; Marshall, L.; Strachan, F.E.; et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart 2019, 105, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Smulders, M.W.; Kietselaer, B.L.J.H.; Wildberger, J.E.; Dagnelie, P.C.; Brunner-La Rocca, H.; Mingels, A.M.A.; van Cauteren, Y.J.M.; Theunissen, R.A.L.J.; Post, M.J.; Schalla, S.; et al. Initial imaging-guided strategy versus routine care in patients with non–ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2019, 74, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Linde, J.J.; Kelbæk, H.; Hansen, T.F.; Sigvardsen, P.E.; Torp-Pedersen, C.; Bech, J.; Heitmann, M.; Nielsen, O.W.; Høfsten, D.; Kühl, J.T.; et al. Coronary CT Angiography in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Björn, R.; Shmuel, C.; Aaron, C.; Ben-Yehuda, O.; Gersh, B.J.; Lembo, N.J.; Brown, W.M., III; Banning, A.P.; Taggart, D.P.; Serruys, P.W.; et al. B-Type Natriuretic Peptide Assessment in Patients Undergoing Revascularization for Left Main Coronary Artery Disease. Circulation 2018, 138, 469–478. [Google Scholar]
- Zhang, C.; Jiang, L.; Xu, L.; Tian, J.; Liu, J.; Zhao, X.; Feng, X.; Wang, D.; Zhang, Y.; Sun, K.; et al. Implications of N-terminal pro-B-type natriuretic peptide in patients with three-vessel disease. Eur. Heart J. 2019, 40, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, D.N.; Aquino, M.; Claessen, B.E.; Baber, U.; Guedeney, P.; Sorrentino, S.; Vogel, B.; de Winter, R.J.; Sweeny, J.; Kovacic, J.C.; et al. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur. Heart J. 2018, 39, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Kalkman, D.N.; Aquino, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Vogel, B.; Sartori, S.; Montalescot, G.; et al. Residual Inflammatory Risk in Patients with Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2401–2409. [Google Scholar] [CrossRef]
- Lattuca, B.; Sy, V.; Nguyen, L.S.; Bernard, M.; Zeitouni, M.; Overtchouk, P.; Yan, Y.; Hammoudi, N.; Ceccaldi, A.; Collet, J.; et al. Copeptin as a prognostic biomarker in acute myocardial infarction. Int. J. Cardiol. 2019, 274, 337–341. [Google Scholar] [CrossRef]
- Camaro, C.; Damman, P. Antithrombotic PreTreatment and Invasive Strategies in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 2578. [Google Scholar] [CrossRef]
- Parodi, G.; Valenti, R.; Bellandi, B.; Migliorini, A.; Marcucci, R.; Comito, V.; Carrabba, N.; Santini, A.; Gensini, G.F.; Abbate, R.; et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J. Am. Coll. Cardiol. 2013, 61, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Montalescot, G.; Bolognese, L.; Dudek, D.; Goldstein, P.; Hamm, C.; Tanguay, J.; Ten Berg, J.M.; Miller, D.L.; Costigan, T.M.; Goedicke, J.; et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N. Engl. J. Med. 2013, 369, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalescot, G.; Collet, J.-P.; Ecollan, P.; Bolognese, L.; Ten Berg, J.; Dudek, D.; Hamm, C.; Widimsky, P.; Tanguay, J.; Goldstein, P.; et al. Effect of prasugrel pre-treatment strategy in patients undergoing percutaneous coronary intervention for NSTEMI: The ACCOAST-PCI study. J. Am. Coll. Cardiol. 2014, 64, 2563–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvain, J.; Rakowski, T.; Lattuca, B.; Liu, Z.; Bolognese, L.; Goldstein, P.; Hamm, C.; Tanguay, J.; ten Berg, J.; Widimsky, P.; et al. Interval from Initiation of Prasugrel to Coronary Angiography in Patients with Non-ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Lindholm, D.; Varenhorst, C.; Cannon, C.P.; Harrington, R.A.; Himmelmann, A.; Maya, J.; Husted, S.; Steg, P.G.; Cornel, J.; Storey, R.F.; et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: Results from the PLATO trial. Eur. Heart J. 2014, 35, 2083–2093. [Google Scholar] [CrossRef]
- Schüpke, S.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef]
- Tarantini, G.; Mojoli, M.; Varbella, F.; Caporale, R.; Rigattieri, S.; Andò, G.; Cirillo, P.; Pierini, S.; Santarelli, A.; Sganzerla, P.; et al. Timing of Oral P2Y12 Inhibitor Administration in Non-ST Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020. [Google Scholar] [CrossRef]
- Almendro-Delia, M.; Gonzalez-Torres, L.; Garcia-Alcantara, Á.; Reina-Toral, A.; Sánchez, J.A.A.; Yañez, J.C.R.; Hidalgo-Urbano, R.; Rubira, J.C.G.; ARIAM-Andalucía Group. Prognostic impact of clopidogrel pretreatment in patients with acute coronary syndrome managed invasively. Am. J. Cardiol. 2015, 115, 1019–1026. [Google Scholar] [CrossRef]
- Dworeck, C.; Redfors, B.; Angerås, O.; Haraldsson, I.; Odenstedt, J.; Ioanes, D.; Petursson, P.; Völz, S.; Persson, J.; Koul, S.; et al. Association of Pretreatment With P2Y12 Receptor Antagonists Preceding Percutaneous Coronary Intervention in Non-ST-Segment Elevation Acute Coronary Syndromes with Outcomes. JAMA Netw. Open 2020, 3, e2018735. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.; Ardissino, D.; de Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Menichelli, M.; Neumann, F.-J.; Ndrepepa, G.; Mayer, K.; Wöhrle, J.; Bernlochner, I.; Richardt, G.; Witzenbichler, B.; Sibbing, D.; Gewalt, S.; et al. Age- and Weight-Adapted Dose of Prasugrel Versus Standard Dose of Ticagrelor in Patients With Acute Coronary Syndromes: Results From a Randomized Trial. Ann. Intern. Med. 2020, 173, 436–444. [Google Scholar] [CrossRef]
- Schnorbus, B.; Daiber, A.; Jurk, K.; Warnke, S.; Koenig, J.; Lackner, K.J.; Münzel, T.; Gori, T. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: A randomized, blinded, parallel study. Eur. Heart J. 2020, 41, 3144–3152. [Google Scholar] [CrossRef]
- Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Della Riva, D.; Bacchi-Reggiani, L.; Smits, P.C.; Vlachojannis, G.J.; Jensen, L.O.; Christiansen, E.H.; Berencsi, K.; et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2496–2507. [Google Scholar] [CrossRef] [Green Version]
- Giustino, G.; Baber, U.; Sartori, S.; Mehran, R.; Mastoris, I.; Kini, A.S.; Sharma, S.K.; Pocock, S.J.; Dangas, G.D. Duration of dual antiplatelet therapy after drug-eluting stent implantation: A systematic review and meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2015, 65, 1298–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; de Rosa, S.; Baber, U.; et al. Efficacy and safety of alirocumab and evolocumab: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Sorrentino, S.; Giustino, G.; Chapelle, C.; Laporte, S.; Claessen, B.E.; Ollier, E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; et al. Indirect Comparison of the Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Network Meta-Analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Baber, U.; Claessen, B.; Aquino, M.; Camaj, A.; Sorrentino, S.; Vogel, B.; Farhan, S.; Faggioni, M.; Chandrasekhar, J.; et al. Temporal trends, determinants, and impact of high-intensity statin prescriptions after percutaneous coronary intervention: Results from a large single-center prospective registry. Am. Heart J. 2019, 207, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Baber, U.; Camaj, A.; Sorrentino, S.; Aquino, M.; Blum, M.; Chandiramani, R.; Goel, R.; Elsayed, S.; et al. Temporal Trends in Statin Prescriptions and Residual Cholesterol Risk in Patients with Stable Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Am. J. Cardiol. 2019, 123, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Bhatt, D.L.; Eikelboom, J.W.; Fox, K.A.A.; Geisler, T.; Gibson, C.M.; Gonzalez-Juanatey, J.R.; James, S.; Lopes, R.D.; Mehran, R.; et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.C.J.; Leadbeater, P.D.; Chan, M.V.; Kirkby, N.S.; Jakubowski, J.A.; Mitchell, J.A.; Warner, T.D. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 2011, 9, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet 2018, 392, 940–949. [Google Scholar] [PubMed]
- Guedeney, P.; Montalescot, G. GLOBAL LEADERS: Looking now at the bigger picture. EuroIntervention 2019, 15, e1030–e1032. [Google Scholar] [CrossRef]
- Hahn, J.-Y.; Song, Y.B.; Oh, J.-H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.; Jeong, J.; Cho, B.R.; Oh, S.K.; et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019, 321, 2428–2437. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Guedeney, P.; Mesnier, J.; Sorrentino, S.; Abcha, F.; Zeitouni, M.; Lattuca, B.; Silvain, J.; de Rosa, S.; Indolfi, C.; Collet, J.; et al. Early Aspirin Discontinuation Following Acute Coronary Syndrome or Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Tersalvi, G.; Biasco, L.; Cioffi, G.M.; Pedrazzini, G. Acute Coronary Syndrome, Antiplatelet Therapy, and Bleeding: A Clinical Perspective. J. Clin. Med. 2020, 9, 2064. [Google Scholar] [CrossRef]
- Claassens, D.M.; Sibbing, D. De-Escalation of Antiplatelet Treatment in Patients with Myocardial Infarction Who Underwent Percutaneous Coronary Intervention: A Review of the Current Literature. J. Clin. Med. 2020, 9, 2983. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef] [Green Version]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; Van’t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef]
- Fontana, P.; Roffi, M.; Reny, J.-L. Platelet Function Test Use for Patients with Coronary Artery Disease in the Early 2020s. J. Clin. Med. 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan Pin Yin, D.; Azzahhafi, J.; James, S. Risk Assessment Using Risk Scores in Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 3039. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [Green Version]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Mehran, R.; Dangas, G.; Baber, U.; Sartori, S.; Chandiramani, R.; Stefanini, G.G.; Angiolillo, D.J.; Capodanno, D.; Urban, P.; et al. Validation of the Academic Research Consortium High Bleeding Risk Definition in Contemporary PCI Patients. J. Am. Coll. Cardiol. 2020, 75, 2711–2722. [Google Scholar] [CrossRef]
- Dewilde, W.J.M.; Oirbans, T.; Verheugt, F.W.A.; Kelder, J.C.; de Smet, B.J.G.L.; Herrman, J.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.C.M.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Bor, W.; Gorog, D.A. Antithrombotic Therapy in Patients with Atrial Fibrillation and Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 2020. [Google Scholar] [CrossRef]
- Zwart, B.; Parker, W.A.E.; Storey, R.F. New Antithrombotic Drugs in Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 2059. [Google Scholar] [CrossRef]
- Limbruno, U.; De Sensi, F.; Cresti, A.; Picchi, A.; Lena, F.; De Caterina, R. Optimal Antithrombotic Treatment of Patients with Atrial Fibrillation Early after an Acute Coronary Syndrome-Triple Therapy, Dual Antithrombotic Therapy with an Anticoagulant… Or, Rather, Temporary Dual Antiplatelet Therapy? J. Clin. Med. 2020, 9, 2673. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Goette, A.; Tijssen, J.; Eckardt, L.; Lewalter, T.; Vranckx, P.; Valgimigli, M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019, 40, 3757–3767. [Google Scholar] [PubMed] [Green Version]
- Yasuda, S.; Kaikita, K.; Akao, M.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; Kimura, K.; Hirayama, A.; et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N. Engl. J. Med. 2019, 381, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
| Trial | Year of Publication | Main Inclusion Criteria | Proportion of Patient with ACS | Evaluated Antithrombotic Regimen | Main Results | Number Needed to Treat (Ischemic Outcomes) | Number Needed to Harm (Bleeding Outcomes) |
|---|---|---|---|---|---|---|---|
| DAPT [53] | 2014 | PCI followed by uncomplicated 12-month DAPT | 4251/9961 (42.7%) | Prolonged DAPT with aspirin and clopidogrel (65.2%) or prasugrel (34.8%) for 18 months | Prolonged DAPT reduced ST (HR 0.29 95%CI0.17–0.48) and MACE (HR 0.71 95%CI0.59–0.85) with increased risk of moderate or severe bleeding | 63 | 105 |
| PEGASUS TIMI 54 [54] | 2015 | - Prior MI within 1 to 3 years - Age > 50 years - At least one feature among: age > 65 years; diabetes mellitus; >1 prior MI; multivessel disease, chronic kidney disease | 21162/21162 (100%) including 3499/21162 (16.6%) patients with multiple prior MI | Prolonged DAPT with aspirin and ticagrelor (60 mg twice daily or 90 mg twice daily) | Both regimen of prolonged DAPT with ticagrelor reduced the risk of CV death, MI or stroke (HR 0.85 95%CI 0.75–0.96 for 90 mg b.i.d. and HR 0.84 95%CI 0.74–0.95 for 60 mg b.i.d.) and increased the risk of TIMI major bleeding (HR 2.69 95%CI 1.96–3.70 for 90 mg b.i.d. and HR 2.32 95%CI 1.68–3.21 for 60 mg b.i.d.) | 84 for ticagrelor 90 mg b.i.d. 79 for ticagrelor 60 mg b.i.d. | 65 for ticagrelor 90 mg b.i.d. 81 for ticagrelor 60 mg b.i.d. |
| COMPASS [55] | 2017 | - Established coronary and/or peripheral artery disease - If coronary disease and age <65 years then at least one of the following: ≥2 vascular bed disease, ≥2 risk factors among: diabetes mellitus, current smocking, chronic kidney disease, heart failure, or prior stroke | 17028/27395 (62.2%) patients with prior MI | Prolonged rivaroxaban 2.5 twice daily and aspirin (100 mg once daily) or rivaroxaban (5 mg twice a day) monotherapy | DAT with rivaroxaban + aspirin was associated with a reduction of CV death, MI or stroke (HR 0.76 95%CI 0.66–0.86) but not rivaroxaban alone (HR 0.90 95%CI 0.79–1.03). Both regimens increased the risk of major bleeding (HR 1.70 95%CI 1.40–2.05 for rivaroxaban + aspirin and HR1.51 95%CI 1.25–1.84 for rivaroxaban alone) | 77 for rivaroxaban + aspirin | 84 for rivaroxaban + aspirin |
| Risk Category | Complex Coronary Lesion and/or Percutaneous Procedure | High Thrombotic Risk | Moderate Thrombotic Risk |
|---|---|---|---|
| Criteria |
|
|
|
| High Bleeding Risk: ≥ 1 Major Criterion or ≥2 Minor Criteria | |
|---|---|
|
|
| Trial | Years of Publication | Main Inclusion Criteria | Proportion of Patient Presenting with ACS | Antithrombotic Regimen Evaluated | Main Results | Number Needed to Prevent One Ischemic Outcome | Number Needed to Prevent One Bleeding Outcome |
|---|---|---|---|---|---|---|---|
| WOEST [58] | 2013 | Indication for Oral Anticoagulation and PCI | 155/563 (27.5%) | DAT (Clopidogrel +VKA) vs. TAT (Aspirin + Clopidogrel + VKA) | Reduced Risk of Bleeding with DAT (HR 0.36 95% CI 0.26–0.50) And MACE (HR 0.60 95% CI 0.38–0.94) | 15 | 4 (For Any Bleeding) 42 (For TIMI Major Bleeding) |
| PIONEER AF-PCI [59] | 2016 | Non-valvular AF and PCI with coronary stent implantation | 1096/2124 (51.6%) | DAT With Rivaroxaban (15 mg Once Daily) + P2Y12 Inhibitors and TAT with Rivaroxaban (2.5 mg Twice Daily) + Aspirin + Clopidogrel Or VKA + Aspirin + Clopidogrel | DAT Was Associated with Reduced Risk of Clinically Significant Bleeding (HR 0.59 95%CI 0.47–0.76) Vs. TAT with VKA + Aspirin, Without Significant Difference in Term of Ischemic Events | - | 10 |
| RE-DUAL PCI [60] | 2017 | Non valvular AF Successful PCI < 120 h | 2007/2725 (73.7%) | TAT With Dabigatran (110 Mg Twice Daily Or 150 mg Twice Daily) + P2Y12 Inhibitors Vs. TAT with VKA + Aspirin+ P2Y12 Inhibitors | Both Regimens of DAT Were Associated with Reduced Risk of ISTH Major or Clinically Relevant Bleeding (110 mg B.I.D. HR 0.52 95%CI 0.42–0.63 And 150 mg B.I.D. HR 0.72 95%CI0.58–0.88) | - | 9 For Dabigatran 110 mg Twice Daily And 18 For 150 mg Twice Daily |
| AUGUSTUS [61] | 2019 | AF and recent PCI or ACS with planned used of at least 6 months of P2Y12 | 2811/4614 (60.9%) | TAT With Apixaban or VKA Vs. DAT With Apixaban Or VKA +Aspirin+P2Y12 Inhibitors | DAT Was Associated with Reduced Risk of ISTH Major or Clinically Relevant Bleeding (HR 0.53 95%CI 0.45–0.63) Without Significant Difference In Term Of Ischemic Events | - | 14 |
| ENTRUST-AF PCI [62] | 2019 | Non valvular AF and PCI procedure for stable CAD or ACS | 777/1506 (51.6%) | DAT With Edoxaban 60 Mg Twice Daily +P2Y12 Inhibitor or TAT With VKA+Aspirin+P2Y12 Inhibitors | DAT Was Not Significantly Associated with Reduced Risk of ISTH Major Or Clinically Relevant Bleeding (HR 0.83 95%CI 0.65–1.05) Without Significant Difference For Ischemic Events | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedeney, P.; Collet, J.-P. Diagnosis and Management of Acute Coronary Syndrome: What is New and Why? Insight From the 2020 European Society of Cardiology Guidelines. J. Clin. Med. 2020, 9, 3474. https://doi.org/10.3390/jcm9113474
Guedeney P, Collet J-P. Diagnosis and Management of Acute Coronary Syndrome: What is New and Why? Insight From the 2020 European Society of Cardiology Guidelines. Journal of Clinical Medicine. 2020; 9(11):3474. https://doi.org/10.3390/jcm9113474
Chicago/Turabian StyleGuedeney, Paul, and Jean-Philippe Collet. 2020. "Diagnosis and Management of Acute Coronary Syndrome: What is New and Why? Insight From the 2020 European Society of Cardiology Guidelines" Journal of Clinical Medicine 9, no. 11: 3474. https://doi.org/10.3390/jcm9113474
APA StyleGuedeney, P., & Collet, J.-P. (2020). Diagnosis and Management of Acute Coronary Syndrome: What is New and Why? Insight From the 2020 European Society of Cardiology Guidelines. Journal of Clinical Medicine, 9(11), 3474. https://doi.org/10.3390/jcm9113474





