Predicting Clinical Outcome with Phenotypic Clusters in COVID-19 Pneumonia: An Analysis of 12,066 Hospitalized Patients from the Spanish Registry SEMI-COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Selection, and Data Collection
2.2. Treatments Prescribed
2.3. Outcomes Definition
2.4. Statistical Analysis
3. Results
3.1. General Data and Symptoms
3.2. Clustering Analysis
3.3. Treatments and Outcomes
3.4. Primary and Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)-China. China CDC Weekly. 2020. Available online: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 (accessed on 12 March 2020).
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, e101. [Google Scholar] [CrossRef]
- Capra, R.; De Rossi, N.; Mattioli, F.; Romanelli, G.; Scarpazza, C.; Sormani, M.P.; Cossi, S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020, 76, 31–35. [Google Scholar] [CrossRef]
- Campochiaro, C.; Della-Torre, E.; Cavalli, G.; De Luca, G.; Ripa, M.; Boffini, N.; Tomelleri, A.; Baldissera, E.; Rovere-Querini, P.; Ruggeri, A.; et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur. J. Intern. Med. 2020, 76, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Morena, V.; Milazzo, L.; Oreni, L.; Bestetti, G.; Fossali, T.; Bassoli, C.; Torre, A.; Cossu, M.V.; Minari, C.; Ballone, E.; et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 2020, 76, 36–42. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19-Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Pagès, J. Principal component methods–hierarchical clustering–partitional clustering: Why would we need to choose for visualizing data? Tech. Rep. 2010, 9, 1–17. [Google Scholar]
- Westreich, D.; Greenland, S. The table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 2013, 177, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.; Vargas-Núñez, J.; et al. Características clínicas de los pacientes hospitalizados con COVID-19 en España: Resultados del Registro SEMI-COVID-19. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with covid-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Sudre, C.H.; Lee, K.; Lochlainn, M.N.; Varsavsky, T.; Murray, B.; Graham, M.; Menni, C.; Modat, M.; Bowyer, R.C.; Nguyen, L.H.; et al. Symptom clusters in Covid19: A potential clinical prediction tool from the COVID Symptom study app. medRxiv 2020. [Google Scholar] [CrossRef]
- Haldar, P.; Berair, R. Endotypes and asthma. In Clinical Asthma. Theory and Practice; Bernstein, J.S., Levy, M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 39–43. [Google Scholar]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Cherian, R.; Chandra, B.; Tung, M.L.; Vuylsteke, A. COVID-19 conundrum: Clinical phenotyping based on pathophysiology as a promising approach to guide therapy in a novel illness. Eur. Respir. J. 2020, 2020, 2002135. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]

| All Patients n = 12,066 | |
|---|---|
| Age yr., median (IQR) | 68 (56–79) |
| Gender, males n (%) | 7052 (58.5) |
| Race | |
| Caucasian | 10,635 (89.5) |
| Black | 43 (0.4) |
| Hispanic | 1041 (8.8) |
| Asian | 59 (0.5) |
| Others | 100 (0.8) |
| BMI, median (IQR) | 28 (25–31) |
| Days from onset to admission, median (IQR) | 7 (4–9) |
| Smoking behavior, n (%) | |
| Never | 8035 (69.7) |
| Current smoker | 567 (4.9) |
| Former smoker | 2930 (25.4) |
| Comorbidity, n (%) | |
| Arterial hypertension | 6030 (50) |
| Diabetes mellitus | 2309 (19.2) |
| Hyperlipidemia | 4741 (39.4) |
| COPD | 786 (6.5) |
| Asthma | 869 (7.2) |
| OSAS | 751 (6.3) |
| Ischemic cardiopathy | 931 (7.7) |
| Chronic heart failure | 809 (6.7) |
| Chronic kidney disease | 696 (5.8) |
| Chronic hepatopathy | 440 (3.7) |
| Active cancer | 1196 (9.9) |
| Autoimmune disease | 277 (2.3) |
| Charlson’s index, median (IQR) | 1 (0–2) |
| All Patients n = 12,066 | C1 n = 8737 | C2 n = 1196 | C3 n = 880 | C4 n = 1253 | p-Value | |
|---|---|---|---|---|---|---|
| Symptoms n (%) | ||||||
| High-grade fever ≥ 38 °C | 7915 (65.6) | 5672 (64.9) | 843 (70.5) | 598 (68) | 802 (64) | <0.001 |
| Low-grade fever < 38 °C | 2431 (20.1) | 1723 (19.7) | 238 (19.9) | 194 (22) | 276 (22) | <0.001 |
| Cough | 9142 (75.8) | 6501 (74.4) | 993 (83) | 766 (87) | 882 (70.4) | <0.001 |
| Dyspnea | 7205 (59.7) | 5340 (61.1) | 727 (60.8) | 492 (55.9) | 646 (51.6) | <0.001 |
| Arthromyalgia | 3794 (31.4) | 2432 (27.8) | 569 (47.6) | 370 (42) | 423 (33.8) | <0.001 |
| Sore throat | 1191 (9.9) | 0 | 186 (15.6) | 880 (100) | 125 (10) | <0.001 |
| Headache | 1402 (11.6) | 730 (8.4) | 292 (24.4) | 202 (23) | 178 (14.2) | <0.001 |
| Anosmia | 879 (7.3) | 0 | 879 (73.5) | 0 | 0 | <0.001 |
| Ageusia | 992 (8.2) | 0 | 988 (82.6) | 0 | 4 (0.3) | <0.001 |
| Diarrhea | 2943 (24.4) | 1654 (18.9) | 473 (39.5) | 181 (20.6) | 635 (50.7) | <0.001 |
| Vomiting | 891 (7.4) | 0 | 110 (9.2) | 0 | 781 (62.3) | <0.001 |
| Abdominal pain | 738 (6.1) | 0 | 79 (6.6) | 0 | 659 (52.6) | <0.001 |
| Heart rate upon admission, bpm median (IQR) | 88 (77–100) | 87 (76–100) | 89 (79–100) | 89 (78–100) | 87 (77–100) | 0.001 |
| Respiratory rate upon admission > 20 bpm, n (%) | 3833 (32.5) | 2939 (34.4) | 304 (26.1) | 249 (28.9) | 341 (28) | <0.001 |
| All Patients n = 12,066 | C1 n = 8737 | C2 n = 1196 | C3 n = 880 | C4 n = 1253 | p-Value | |
|---|---|---|---|---|---|---|
| Age yr, median (IQR) | 68 (56–79) | 70 (57–80) | 61 (51–71) | 64 (52–75) | 67 (53–77) | <0.001 |
| Gender, males n (%) | 7052 (58.5) | 5303 (60.8) | 643 (53.8) | 507 (57.6) | 599 (47.9) | <0.001 |
| Race | ||||||
| Caucasian | 10,635 (89.5) | 7820 (90.9) | 1023 (86.7) | 738 (84.7) | 1054 (86) | |
| Black | 43 (0.4) | 35 (0.4) | 3 (0.3) | 1 (0.1) | 4 (0.3) | |
| Hispanic | 1041 (8.8) | 643 (7.5) | 137 (11.6) | 117 (13.4) | 144 (11.7) | <0.001 |
| Asian | 59 (0.5) | 41 (0.5) | 2 (0.2) | 6 (0.7) | 10 (0.8) | |
| Others | 100 (0.8) | 62 (9.7) | 15 (1.3) | 9 (1) | 14 (1.1) | |
| BMI, median (IQR) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 28 (25–31) | 0.426 |
| Days from onset to admission, median (IQR) | 7 (4–9) | 6 (3–9) | 8 (6–10) | 7 (4–10) | 7 (4–9) | <0.001 |
| Smoking behavior, n (%) | ||||||
| Never | 8035 (69.7) | 5761 (69.2) | 793 (68.7) | 587 (69.4) | 894 (74.3) | |
| Current smoker | 567 (4.9) | 414 (5) | 64 (5.5) | 41 (4.8) | 48 (4) | 0.027 |
| Former smoker | 2930 (25.4) | 2153 (25.9) | 297 (25.7) | 218 (25.8) | 262 (21.8) | |
| Comorbidity, n (%) | ||||||
| Arterial hypertension | 6030 (50) | 4571 (52.4) | 468 (39.1) | 386 (43.9) | 605 (48.4) | <0.001 |
| Diabetes mellitus | 2309 (19.2) | 1774 (20.4) | 177 (14.8) | 156 (17.8) | 202 (16.2) | <0.001 |
| Hyperlipidemia | 4741 (39.4) | 3527 (40.4) | 420 (35.1) | 325 (37) | 469 (37.5) | 0.001 |
| COPD | 786 (6.5) | 649 (7.4) | 44 (3.7) | 43 (4.9) | 50 (4) | <0.001 |
| Asthma | 869 (7.2) | 630 (7.2) | 90 (7.5) | 57 (6.5) | 92 (7.4) | 0.827 |
| OSAS | 751 (6.3) | 574 (6.6) | 57 (4.8) | 48 (5.5) | 72 (5.8) | 0.057 |
| Ischemic cardiopathy | 931 (7.7) | 722 (8.3) | 49 (4.1) | 65 (7.4) | 95 (7.6) | <0.001 |
| Chronic heart failure | 809 (6.7) | 660 (7.6) | 41 (3.4) | 42 (4.8) | 66 (5.3) | <0.001 |
| Chronic kidney disease | 696 (5.8) | 550 (6.3) | 36 (3) | 36 (4.1) | 74 (5.9) | <0.001 |
| Chronic hepatopathy | 440 (3.7) | 330 (3.8) | 46 (3.8) | 22 (2.5) | 42 (3.4) | <0.001 |
| Active cancer | 1196 (9.9) | 916 (10.5) | 94 (7.9) | 72 (8.2) | 114 (9.1) | 0.005 |
| Autoimmune disease | 277 (2.3) | 195 (2.2) | 33 (2.8) | 19 (2.2) | 30 (2.4) | 0.701 |
| Charlson’s index, median (IQR) | 1 (0–2) | 1 (0–2) | 0 (0,1) | 0 (0,1) | 0 (0–2) | <0.001 |
| PaO2/FiO2 at admission, mmHg median (95%CI) | 294 (292–296) | 289 (287–292) | 311 (306–317) | 305 (298–312) | 301 (296–307) | <0.001 |
| Lab test upon admission, median (IQR) | ||||||
| Lymphocytes ×106/L | 910 (680–1280) | 900 (660–1270) | 1000 (700–1310) | 1000 (715–1300) | 900 (630–1210) | <0.001 |
| CRP mg/L | 74 (30–141) | 78 (30–146) | 69 (29–130) | 63 (26–135) | 66 (27–129) | <0.001 |
| LDH U/L | 329 (253-444) | 332 (255–450) | 309 (247–412) | 330 (248–446) | 331 (256–439) | <0.001 |
| ALT U/L | 30 (19-47) | 29 (19–46) | 32 (21–52) | 31 (21–49) | 30 (20–48) | <0.001 |
| Ferritin mcg/L | 655 (324-1281) | 669 (330–1320) | 634 (291–1172) | 587 (310–1167) | 620 (326–1265) | 0.051 |
| D-dimer ng/mL | 654 (370-1204) | 680 (382–1290) | 594 (346–980) | 595 (347–1023) | 608 (350–1152) | <0.001 |
| All Patients n = 12,066 | C1 n = 8737 | C2 n = 1196 | C3 n = 880 | C4 n = 1253 | p-Value | |
|---|---|---|---|---|---|---|
| HCQ, n (%) | 10,665 (88.6) | 7654 (87.9) | 1130 (94.5) | 770 (87.6) | 1111 (88.8) | <0.001 |
| LPV/r, n (%) | 7894 (65.7) | 5640 (64.8) | 783 (65.5) | 610 (69.5) | 861 (69) | 0.002 |
| Azithromicin, n (%) | 7558 (62.9) | 5407 (62.2) | 835 (69.8) | 510 (58) | 806 (64.5) | <0.001 |
| Remdesivir, n (%) | 60 (0.5) | 36 (0.4) | 10 (0.8) | 5 (0.6) | 9 (0.7) | 0.150 |
| Interferon, n (%) | 1496 (12,5) | 1122 (13) | 68 (5.7) | 141 (16.1) | 165 (13.2) | <0.001 |
| Tocilizumab, n (%) | 1121 (9.3) | 810 (9.3) | 110 (9.2) | 93 (10.6) | 108 (8.7) | 0.487 |
| Corticosteroids, n (%) | 4343 (36.2) | 3254 (37.5) | 399 (33.5) | 273 (31.2) | 417 (33.4) | <0.001 |
| Heparin, n (%) | <0.001 | |||||
| Prophylactic LMWH | 7903 (65.9) | 5633 (65) | 817 (68.5) | 584 (66.6) | 869 (69.7) | |
| Middle doses LMWH | 815 (6.8) | 589 (6.8) | 97 (8.1) | 49 (5.6) | 80 (6.4) | |
| High doses LMWH | 1305 (10.9) | 997 (11.5) | 120 (10.1) | 90 (10.3) | 98 (7.9) | |
| Oral anticoagulation, n (%) | 0.004 | |||||
| Oral anti-vitamin K drugs | 189 (1.6) | 156 (1.8) | 10 (0.8) | 7 (0.8) | 16 (1.3) | |
| DOACs | 195 (1.6) | 157 (1.8) | 10 (0.8) | 10 (1.1) | 18 (1.4) |
| All Patients n = 12,066 | C1 n = 8737 | C2 n = 1196 | C3 n = 880 | C4 n = 1253 | p-Value | |
|---|---|---|---|---|---|---|
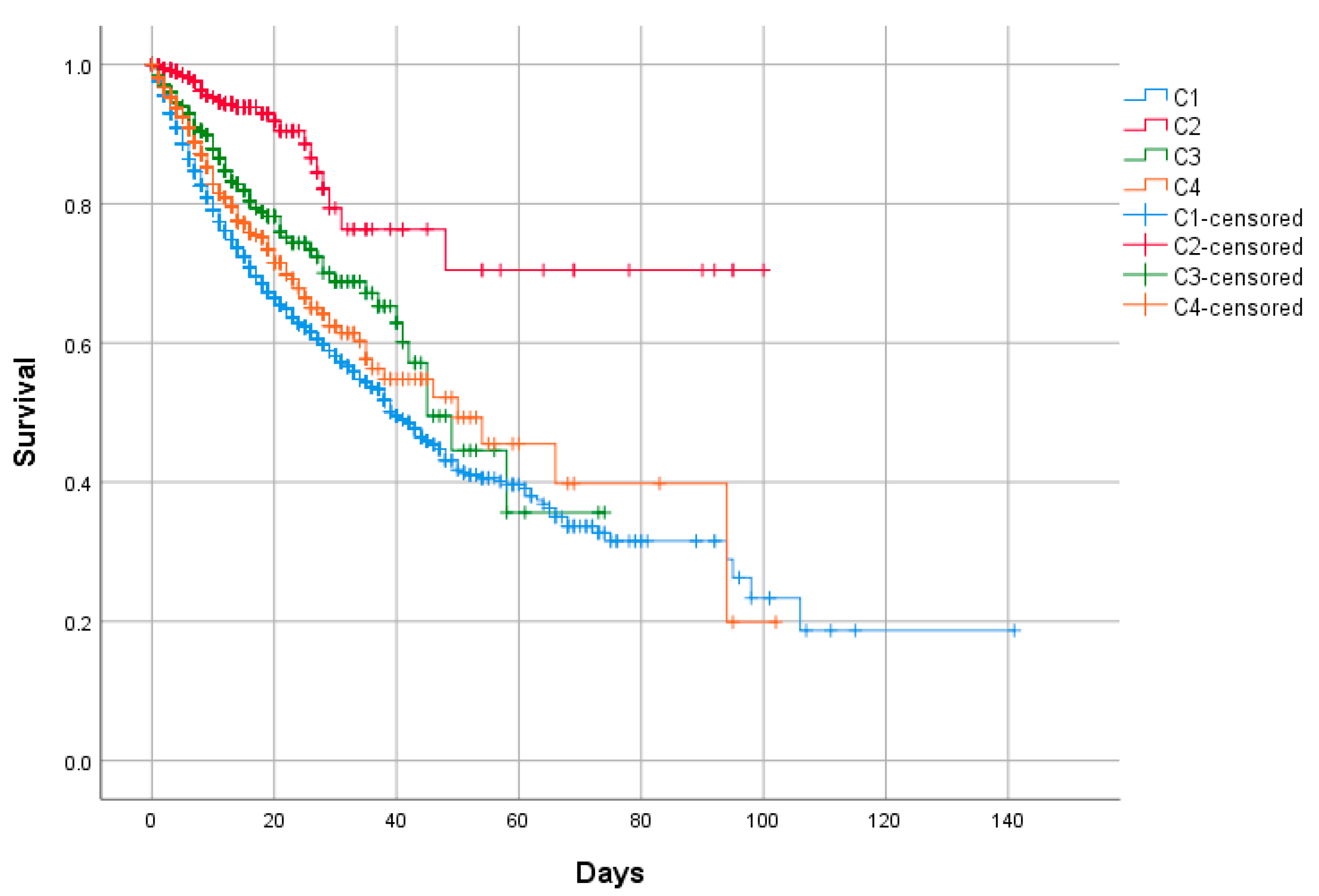
| Death, n (%) | 2522 (20.9) | 2109 (24.1) | 51 (4.3) | 129 (14.7) | 233 (18.6) | <0.001 |
| Length of stay, days mean (range) | 11.3 (1–141) | 11.6 (1–141) | 9.6 (1–100) | 11.4 (1–74) | 11.4 (1–102) | 0.407 |
| Oxygenation/ventilation, n (%) | ||||||
| HFNC | 1038 (8.7) | 757 (8.8) | 82 (6.9) | 75 (8.5) | 124 (10) | 0.053 |
| NIMV | 641 (5.3) | 485 (5.6) | 46 (3.9) | 44 (5) | 66 (5.3) | 0.094 |
| IMV | 906 (7.5) | 694 (8) | 49 (4.1) | 75 (8.6) | 88 (7.1) | <0.001 |
| ICU admission, n (%) | 1120 (9.3) | 847 (9.7) | 71 (5.9) | 95 (10.8) | 107 (8.5) | <0.001 |
| Univariate Analysis OR (95%CI) | p-Value | Multivariate Analysis * OR (95%CI) | p-Value | Multivariate Analysis ** OR (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| Age/year | 1.09 (1.09–1.10) | <0.001 | 1.08 (1.07–1.08) | <0.001 | 1.09 (1.07–1.09) | <0.001 |
| Gender (female) | 0.78 (0.71–0.86) | <0.001 | 0.64 (0.59–0.70) | <0.001 | 0.62 (0.51–0.75) | <0.001 |
| BMI | 1.02 (1.01–1.04) | <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Clusters | ||||||
| C1 | 1 ref. | 1 ref. | 1 ref. | |||
| C2 | 0.14 (0.11–0.19) | <0.001 | 0.22 (0.18–0.27) | <0.001 | 0.22 (0.14–0.34) | <0.001 |
| C3 | 0.54 (0.45–0.66) | <0.001 | 0.57 (0.48–0.67) | <0.001 | 0.56 (0.37–0.83) | 0.004 |
| C4 | 0.72 (0.62–0.84) | <0.001 | 1.15 (1.01–1.31) | 0.035 | 1.15 (0.85–1.54) | 0.362 |
| Comorbidity | ||||||
| Arterial hypertension | 3.07 (2.79–3.38) | <0.001 | 1.13 (1.04–1.23) | 0.006 | NS | |
| Diabetes mellitus | 2.07 (1.87–2.29) | <0.001 | NS | NS | ||
| Hyperlipidemia | 1.80 (1.64–1.96) | <0.001 | NS | NS | ||
| COPD | 2.82 (2.43–3.27) | <0.001 | 1.36 (1.21–1.53) | <0.001 | 1.36 (1.04–1.78) | 0.024 |
| Ischemic cardiopathy | 2.67 (2.32–3.07) | <0.001 | 1.19 (1.06–1.34) | 0.005 | NS | |
| Chronic heart failure | 3.74 (3.23–4.32) | <0.001 | 1.16 (1.02–1.32) | 0.027 | NS | |
| Chronic kidney disease | 3.18 (2.72–3.72) | <0.001 | NS | NS | ||
| Chronic hepatopathy | 1.57 (1.27–1.94) | <0.001 | 1.20 (1.00–1.44) | 0.048 | NS | |
| Active cancer | 2.23 (1.96–2.53) | <0.001 | NS | NS | ||
| Charlson’s index | 1.37 (1.34–1.41) | <0.001 | 1.18 (1.15–1.20) | <0.001 | 1.20 (1.14–1.25) | <0.001 |
| Heart rate upon admission | 1.00 (0.99–1.00) | 0.278 | 1.01 (1.01–1.01) | <0.001 | ||
| Respiratory rate upon admission > 20 bpm | 4.48 (4.08–4.92) | <0.001 | 2.88 (2.66–3.11) | <0.001 | 3.09 (2.59–3.70) | <0.001 |
| PaO2/FiO2 upon admission | 0.99 (0.99–0.99) | <0.001 | 0.99 (0.99–0.99) | <0.001 | 0.99 (0.99–0.99) | <0.001 |
| Lab test upon admission | ||||||
| Lymphocytes ×106/L | 1.00 (1.00–1.00) | 0.768 | NS | |||
| CRP mg/L | 1.01 (1.01.1.01) | <0.001 | 1.01 (1.01.1.01) | <0.001 | NS | |
| LDH U/L | 1.00 (1.00–1.00) | <0.001 | 1.01 (1.01.1.01) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| ALT U/L | 1.00 (0.99–1.00) | 0.792 | NS | |||
| Ferritin mcg/L | 1.00 (1.00–1.00) | <0.001 | NS | NS | ||
| D–dimer ng/mL | 1.00 (1.00–1.00) | <0.001 | NS | NS | ||
| Treatments during admission | ||||||
| Remdesivir | 1.16 (0.64–2.12) | 0.623 | NS | |||
| Tocilizumab | 1.24 (1.07–1.43) | 0.004 | 1.66 (1.47–1.88) | <0.001 | 1.71 (1.29–2.25) | <0.001 |
| Corticosteroids | 2.06 (1.89–2.26) | <0.001 | 1.21 (1.11–1.31) | <0.001 | 1.24 (1.04–1.49) | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Rivas, M.; Corbella, X.; Mora-Luján, J.M.; Loureiro-Amigo, J.; López Sampalo, A.; Yera Bergua, C.; Esteve Atiénzar, P.J.; Díez García, L.F.; Gonzalez Ferrer, R.; Plaza Canteli, S.; et al. Predicting Clinical Outcome with Phenotypic Clusters in COVID-19 Pneumonia: An Analysis of 12,066 Hospitalized Patients from the Spanish Registry SEMI-COVID-19. J. Clin. Med. 2020, 9, 3488. https://doi.org/10.3390/jcm9113488
Rubio-Rivas M, Corbella X, Mora-Luján JM, Loureiro-Amigo J, López Sampalo A, Yera Bergua C, Esteve Atiénzar PJ, Díez García LF, Gonzalez Ferrer R, Plaza Canteli S, et al. Predicting Clinical Outcome with Phenotypic Clusters in COVID-19 Pneumonia: An Analysis of 12,066 Hospitalized Patients from the Spanish Registry SEMI-COVID-19. Journal of Clinical Medicine. 2020; 9(11):3488. https://doi.org/10.3390/jcm9113488
Chicago/Turabian StyleRubio-Rivas, Manuel, Xavier Corbella, José María Mora-Luján, Jose Loureiro-Amigo, Almudena López Sampalo, Carmen Yera Bergua, Pedro Jesús Esteve Atiénzar, Luis Felipe Díez García, Ruth Gonzalez Ferrer, Susana Plaza Canteli, and et al. 2020. "Predicting Clinical Outcome with Phenotypic Clusters in COVID-19 Pneumonia: An Analysis of 12,066 Hospitalized Patients from the Spanish Registry SEMI-COVID-19" Journal of Clinical Medicine 9, no. 11: 3488. https://doi.org/10.3390/jcm9113488
APA StyleRubio-Rivas, M., Corbella, X., Mora-Luján, J. M., Loureiro-Amigo, J., López Sampalo, A., Yera Bergua, C., Esteve Atiénzar, P. J., Díez García, L. F., Gonzalez Ferrer, R., Plaza Canteli, S., Pérez Piñeiro, A., Cortés Rodríguez, B., Jorquer Vidal, L., Pérez Catalán, I., León Téllez, M., Martín Oterino, J. Á., Martín González, M. C., Serrano Carrillo de Albornoz, J. L., García Sardon, E., ... Gómez-Huelgas, R. (2020). Predicting Clinical Outcome with Phenotypic Clusters in COVID-19 Pneumonia: An Analysis of 12,066 Hospitalized Patients from the Spanish Registry SEMI-COVID-19. Journal of Clinical Medicine, 9(11), 3488. https://doi.org/10.3390/jcm9113488






