Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies
Abstract
1. Introduction
2. Experimental Section
2.1. PMN Isolation and Purification
2.2. DMT Drugs
2.3. Evaluation of Neutrophil Intracellular Antimicrobial Activity
2.4. Oxidative Burst Assay
2.5. Analysis of PMN Apoptosis and Surface Markers by Flow Cytometry
2.6. Quantification of Cytokine Production
2.7. Data Analysis
3. Results
3.1. Direct Effect of DMTs on PMN Intracellular Killing Activity of Klebsiella pneumoniae In vitro
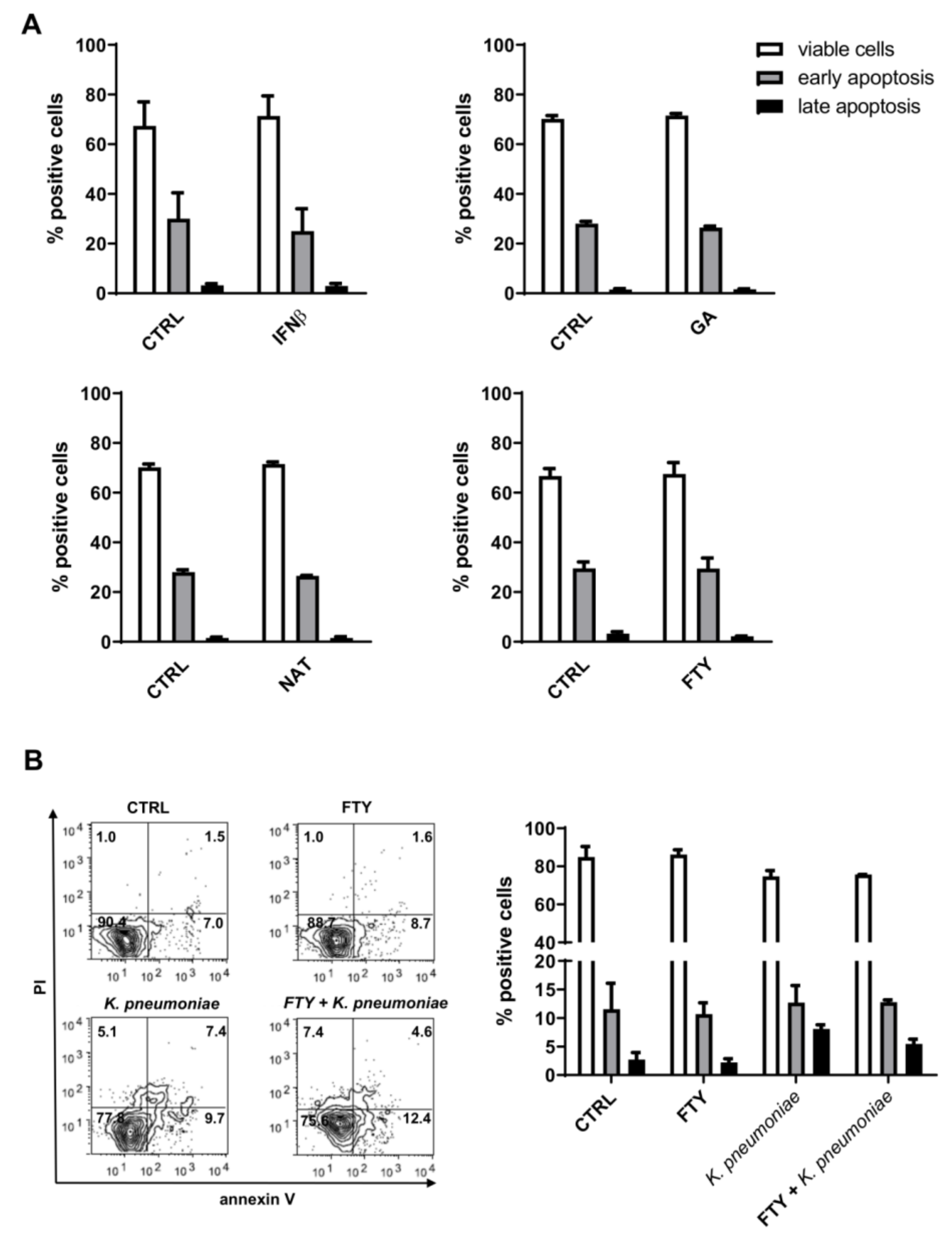
3.2. Effect of DMTs on Apoptosis
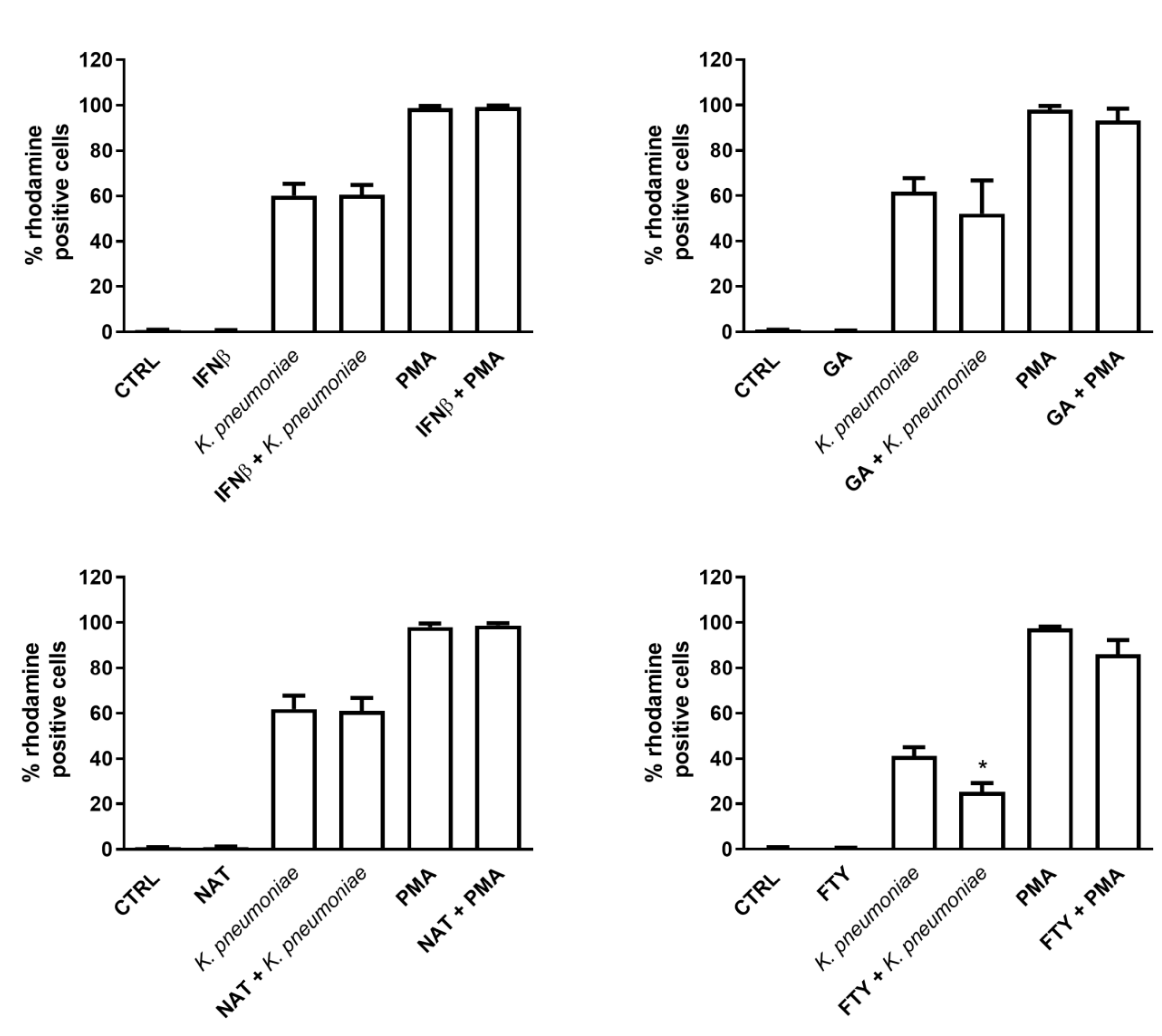
3.3. Effect of DMTs on ROS Production in Resting versus Stimulated PMNs
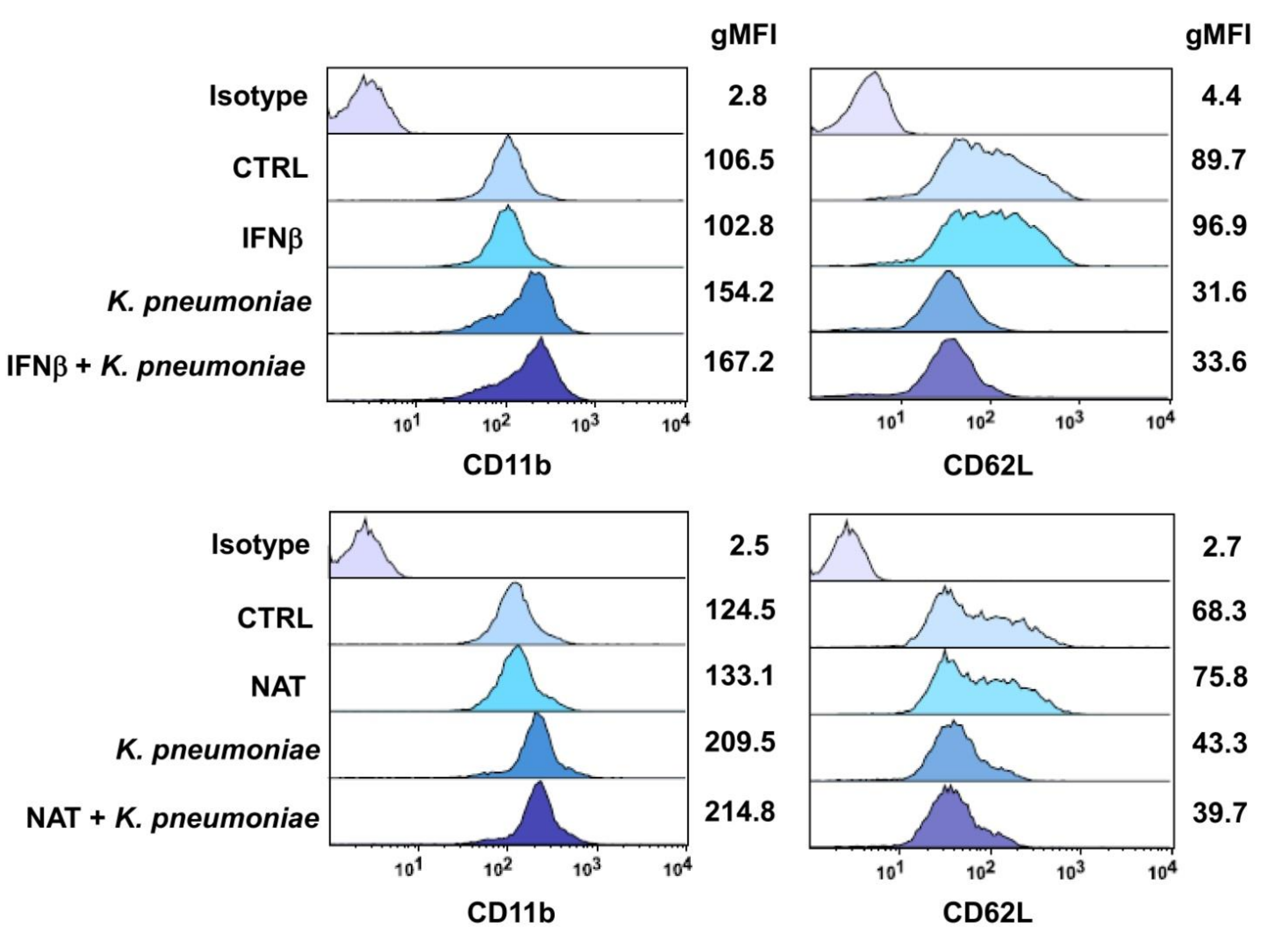
3.4. Effect of DMTs on the Expression of the Neutrophil Adhesion Molecules CD11b and CD62L
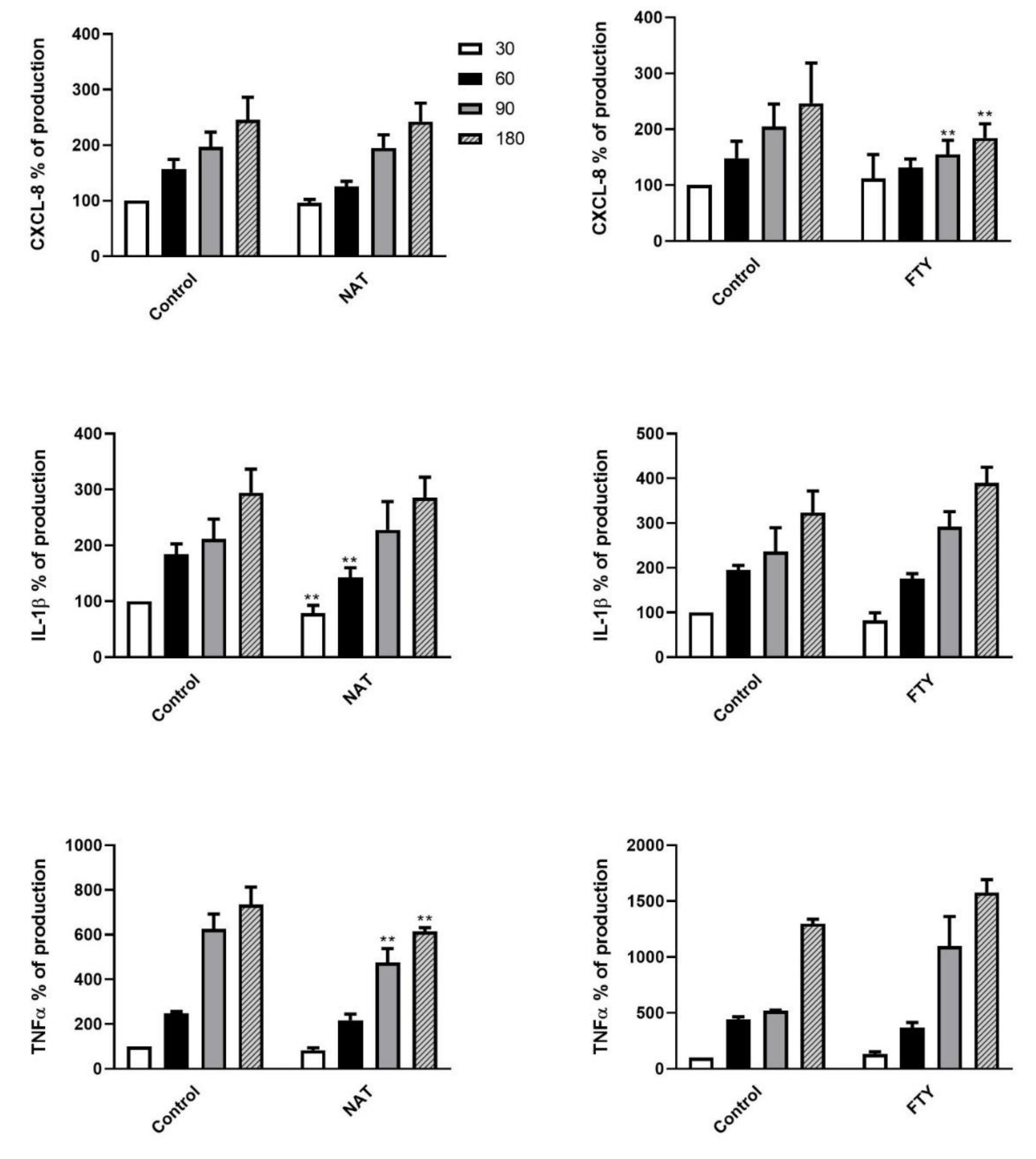
3.5. Cytokine Release Pattern of DMT Pretreated PMNs upon K. pneumoniae Stimulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grebenciucova, E.; Pruitt, A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Curr. Neurol. Neurosci. Rep. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Alping, P.; Burman, J.; Fink, K.; Fogdell-Hahn, A.; Gunnarsson, M.; Hillert, J.; Langer-Gould, A.; Lycke, J.; Nilsson, P.; et al. Infection Risks Among Patients with Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020, 77, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.A. Current therapeutic options in pediatric multiple sclerosis. Curr. Treat. Options Neurol. 2011, 13, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Bichuetti, D.B.; Franco, C.A.; Elias, I.; Mendonça, A.C.R.; Carvalho, L.F.D.; Diniz, D.S.; Tur, C.; Tintoré, M.; Oliveira, E.M.L. Multiple sclerosis risk perception and acceptance for Brazilian patients. Arq. Neuro Psiquiatr. 2018, 76, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Celius, E.G. Infections in patients with multiple sclerosis: Implications for disease-modifying therapy. Acta Neurol. Scand. 2017, 13, 34–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rommer, P.S.; Milo, R.; Han, M.H.; Satyanarayan, S.; Sellner, J.; Hauer, L.; Illes, Z.; Warnke, C.; Laurent, S.; Weber, M.S.; et al. Immunological Aspects of Approved MS Therapeutics. Front. Immunol. 2019, 10, 1564. [Google Scholar] [CrossRef]
- Comi, G.; Radaelli, M.; Soelberg Sørensen, P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017, 389, 1347–1356. [Google Scholar] [CrossRef]
- Ontaneda, D.; Tallantyre, E.; Kalincik, T.; Planchon, S.M.; Evangelou, N. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol. 2019, 18, 973–980. [Google Scholar] [CrossRef]
- Hauser, S.L.; Chan, J.R.; Oksenberg, J.R. Multiple sclerosis: Prospects and promise. Ann. Neurol. 2013, 74, 317–327. [Google Scholar] [CrossRef]
- Rafiee Zadeh, A.; Askari, M.; Azadani, N.N.; Ataei, A.; Ghadimi, K.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 95–104. [Google Scholar] [PubMed]
- Rafiee Zadeh, A.; Ghadimi, K.; Ataei, A.; Askari, M.; Sheikhinia, N.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 2. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 105–114. [Google Scholar] [PubMed]
- Winkelmann, A.; Loebermann, M.; Reisinger, E.C.; Hartung, H.P.; Zettl, U.K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat. Rev. Neurol. 2016, 12, 217–233. [Google Scholar] [CrossRef]
- Rissanen, E.; Remes, K.; Airas, L. Severe neutropenia after rituximab-treatment of multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 3–5. [Google Scholar] [CrossRef]
- Fierro, M.T.; Cuffini, A.M.; Novelli, M.; Banche, G.; Allizond, V.; Comessatti, A.; Brizio, M.; Scalas, D.; Merlino, C.; Quaglino, P.; et al. Functional and phenotypical alterations of polymorphonuclear cells in Sézary syndrome patients. Eur. J. Dermatol. 2011, 21, 921–929. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Naegele, M.; Tillack, K.; Reinhardt, S.; Schippling, S.; Martin, R.; Sospedra, M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J. Neuroimmunol. 2012, 242, 60–71. [Google Scholar] [CrossRef]
- Pliyev, B.K.; Dimitrieva, T.V.; Savchenko, V.G. Cytokine-mediated induction of MHC class II in human neutrophils is dependent on NADPH oxidase activity. Eur. J. Cell Biol. 2015, 94, 67–70. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Allizond, V.; Scutera, S.; Rossi, S.; Musso, T.; Crocillà, C.; Cavalla, P.; Trebini, C.; Marra, E.S.; Cuffini, A.M.; Banche, G. Polymorphonuclear Cell Functional Impairment in Relapsing Remitting Multiple Sclerosis Patients: Preliminary Data. PLoS ONE 2015, 10, e0131557. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Tullio, V.; Allizond, V.; Mandras, N.; Roana, J.; Scalas, D.; El Fassi, F.; D′Antico, S.; Cuffini, A.M.; Carlone, N. Synergistic effect of erythromycin on polymorphonuclear cell antibacterial activity against erythromycin-resistant phenotypes of Streptococcus pyogenes. Int. J. Antimicrob. Agents 2010, 36, 319–323. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Giacchino, F.; Mandras, N.; Roana, J.; Bonello, F.; Belardi, P.; Tullio, V.; Merlino, C.; Carlone, N.; et al. Effect of dialysis membrane biocompatibility on Polymorphonuclear granulocyte activity in dialysis patients. Nephrol. Dial. Transplant. 2006, 21, 3532–3538. [Google Scholar] [CrossRef][Green Version]
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kröger, A.; Weiss, S.; Jablonska, J. Delayed apoptosis of tumor associated neutrophils in the absence of endogenous IFN-β. Int. J. Cancer 2015, 136, 572–583. [Google Scholar] [CrossRef]
- Pul, R.; Morbiducci, F.; Škuljec, J.; Skripuletz, T.; Singh, V.; Diederichs, U.; Garde, N.; Voss, E.V.; Trebst, C.; Stangel, M. Glatiramer acetate increases phagocytic activity of human monocytes in vitro and in multiple sclerosis patients. PLoS ONE 2012, 7, e51867. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Bao, F.; Cepinskas, G.; Weaver, L.C. Anti-alpha4beta1 integrin antibody induces receptor internalization and does not impair the function of circulating neutrophilic leukocytes. Inflamm. Res. 2010, 59, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fields, A.J.; Kapteijn, B.A.; McDonald, J.A. The role of alpha 4 beta 1 integrin in cell motility and fibronectin matrix assembly. J. Cell Sci. 1995, 108, 821–829. [Google Scholar]
- Skrzeczyńska-Moncznik, J.; Bzowska, M.; Nogieć, A.; Sroka, A.; Zarębski, M.; Vallières, L.; Guzik, K. Rapid externalization of 27-kDa heat shock protein (HSP27) and atypical cell death in neutrophils treated with the sphingolipid analog drug FTY720. J. Leukoc. Biol. 2015, 98, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Smalley, D.M.; Ley, K. L-selectin: Mechanisms and physiological significance of ectodomain cleavage. J. Cell Mol. Med. 2005, 9, 255–266. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Nessler, S.; Zhou, D.; Kieseier, B.; Hartung, H.P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Clin. Pract. Neurol. 2006, 2, 201–211. [Google Scholar] [CrossRef]
- Brück, W.; Gold, R.; Lund, B.T.; Oreja-Guevara, C.; Prat, A.; Spencer, C.M.; Steinman, L.; Tintoré, M.; Vollmer, T.L.; Weber, M.S.; et al. Therapeutic decisions in multiple sclerosis: Moving beyond efficacy. JAMA Neurol. 2013, 70, 1315–1324. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef]
- Maffione, A.B.; Tatò, E.; Losito, S.; Nacci, C.; Mitolo, V.; Troiano, M.; Ruggieri, M.; Livrea, P.; Jirillo, E. In vivo effects of recombinant-interferon-beta1b treatment on polymorphonuclear cell and monocyte functions and on T-cell-mediated antibacterial activity in patients with relapsing-remitting multiple sclerosis. Immunopharmacol. Immunotoxicol. 2000, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.S.; Starck, M.; Wagenpfeil, S.; Meinl, E.; Hohlfeld, R.; Farina, C. Multiple sclerosis: Glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain 2004, 127, 1370–1378. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef]
- Arlt, O.; Schwiebs, A.; Japtok, L.; Rüger, K.; Katzy, E.; Kleuser, B.; Radeke, H.H. Sphingosine-1-phosphate modulates dendritic cell function: Focus on non-migratory effects in vitro and in vivo. Cell Physiol. Biochem. 2014, 34, 27–44. [Google Scholar] [CrossRef]
- Sica, F.; Centonze, D.; Buttari, F. Fingolimod Immune Effects Beyond Its Sequestration Ability. Neurol. Ther. 2019, 8, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.M.; del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef]
- Gorlino, C.V.; Ranocchia, R.P.; Harman, M.F.; García, I.A.; Crespo, M.I.; Morón, G.; Maletto, B.A.; Pistoresi-Palencia, M.C. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J. Immunol. 2014, 193, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Kyles, A.E.; Gregory, C.R. In vitro evaluation of the effect of a novel immunosuppressive agent, FTY720, on the function of feline neutrophils. Am. J. Vet. Res. 2006, 67, 588–592. [Google Scholar] [CrossRef]
- Takasaki, T.; Hagihara, K.; Satoh, R.; Sugiura, R. More than Just an Immunosuppressant: The Emerging Role of FTY720 as a Novel Inducer of ROS and Apoptosis. Oxid. Med. Cell Longev. 2018, 2018, 4397159. [Google Scholar] [CrossRef]
- Ahmed, N.; Linardi, D.; Decimo, I.; Mehboob, R.; Gebrie, M.A.; Innamorati, G.; Luciani, G.B.; Faggian, G.; Rungatscher, A. Characterization and Expression of Sphingosine 1-Phosphate Receptors in Human and Rat Heart. Front. Pharmacol. 2017, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Min, X.; Xiao, S.H.; Johnstone, S.; Romanow, W.; Meininger, D.; Xu, H.; Liu, J.; Dai, J.; An, S.; et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 2013, 21, 798–809. [Google Scholar] [CrossRef]
- Kim, S.H.; Uuganbayar, U.; Trinh, H.K.T.; Pham, D.L.; Kim, N.; Kim, M.; Sohn, H.; Park, H.S. Evaluation of Neutrophil Activation Status According to the Phenotypes of Adult Asthma. Allergy Asthma Immunol. Res. 2019, 11, 381–393. [Google Scholar] [CrossRef]
- Trenova, A.G.; Slavov, G.S.; Manova, M.G.; Draganaova-Filipova, M.N.; Mateva, N.G.; Miteva, L.D.; Stanilova, S.A. Alterations in serum levels of IL-17 in contrast to TNF-alpha correspond to disease-modifying treatment in relapsing-remitting multiple sclerosis. Scand. J. Clin. Lab. Investig. 2017, 77, 283–288. [Google Scholar] [CrossRef]
- Lund, B.T.; Ashikian, N.; Ta, H.Q.; Chakryan, Y.; Manoukian, K.; Groshen, S.; Gilmore, W.; Cheema, G.S.; Stohl, W.; Burnett, M.E.; et al. Increased CXCL8 (IL-8) expression in Multiple Sclerosis. J. Neuroimmunol. 2004, 155, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Siglienti, I.; Grauer, O.; Magnus, T.; Scarlato, G.; Toyka, K. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate. J. Neuroimmunol. 2004, 148, 63–73. [Google Scholar] [CrossRef]
- Burger, D.; Molnarfi, N.; Weber, M.S.; Brandt, K.J.; Benkhoucha, M.; Gruaz, L.; Chofflon, M.; Zamvil, S.S.; Lalive, P.H. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Version 2. Proc. Natl. Acad. Sci. USA 2009, 106, 4355–4359. [Google Scholar] [CrossRef]
- Mellergård, J.; Edström, M.; Vrethem, M.; Ernerudh, J.; Dahle, C. Natalizumab treatment in multiple sclerosis: Marked decline of chemokines and cytokines in cerebrospinal fluid. Mult. Scler. 2010, 16, 208–217. [Google Scholar] [CrossRef]
- O′Connell, K.E.; Mok, T.; Sweeney, B.; Ryan, A.M.; Dev, K.K. The use of cytokine signature patterns: Separating drug naïve, interferon and natalizumab-treated multiple sclerosis patients. Autoimmunity 2014, 47, 505–511. [Google Scholar] [CrossRef]
- Pechkovsky, D.V.; Potapnev, M.P.; Zalutskaya, O.M. Different patterns of cytokine regulation of phagocytosis and bacterial killing by human neutrophils. Int. J. Antimicrob Agents 1996, 7, 33–40. [Google Scholar] [CrossRef]
- Crawford, M.A.; Margulieux, K.R.; Singh, A.; Nakamoto, R.K.; Hughes, M.A. Mechanistic insights and therapeutic opportunities of antimicrobial chemokines. Semin. Cell Dev. Biol. 2019, 88, 119–128. [Google Scholar] [CrossRef]
- Wijnands, J.M.A.; Zhu, F.; Kingwell, E.; Fisk, J.D.; Evans, C.; Marrie, R.A.; Zhao, Y.; Tremlett, H. Disease-modifying drugs for multiple sclerosis and infection risk: A cohort study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1050–1056. [Google Scholar] [CrossRef]
- Montgomery, S.; Hillert, J.; Bahmanyar, S. Hospital admission due to infections in multiple sclerosis patients. Eur. J. Neurol. 2013, 20, 1153–1160. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutera, S.; Musso, T.; Cavalla, P.; Piersigilli, G.; Sparti, R.; Comini, S.; Vercellino, M.; Cuffini, A.M.; Banche, G.; Allizond, V. Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies. J. Clin. Med. 2020, 9, 3542. https://doi.org/10.3390/jcm9113542
Scutera S, Musso T, Cavalla P, Piersigilli G, Sparti R, Comini S, Vercellino M, Cuffini AM, Banche G, Allizond V. Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies. Journal of Clinical Medicine. 2020; 9(11):3542. https://doi.org/10.3390/jcm9113542
Chicago/Turabian StyleScutera, Sara, Tiziana Musso, Paola Cavalla, Giorgia Piersigilli, Rosaria Sparti, Sara Comini, Marco Vercellino, Anna Maria Cuffini, Giuliana Banche, and Valeria Allizond. 2020. "Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies" Journal of Clinical Medicine 9, no. 11: 3542. https://doi.org/10.3390/jcm9113542
APA StyleScutera, S., Musso, T., Cavalla, P., Piersigilli, G., Sparti, R., Comini, S., Vercellino, M., Cuffini, A. M., Banche, G., & Allizond, V. (2020). Inhibition of Human Neutrophil Functions In Vitro by Multiple Sclerosis Disease-Modifying Therapies. Journal of Clinical Medicine, 9(11), 3542. https://doi.org/10.3390/jcm9113542







