Impact of Adjuvant Radiotherapy on Survival Outcomes in Intermediate-Risk, Early-Stage Cervical Cancer: Analyses Regarding Surgical Approach of Radical Hysterectomy
Abstract
:1. Introduction
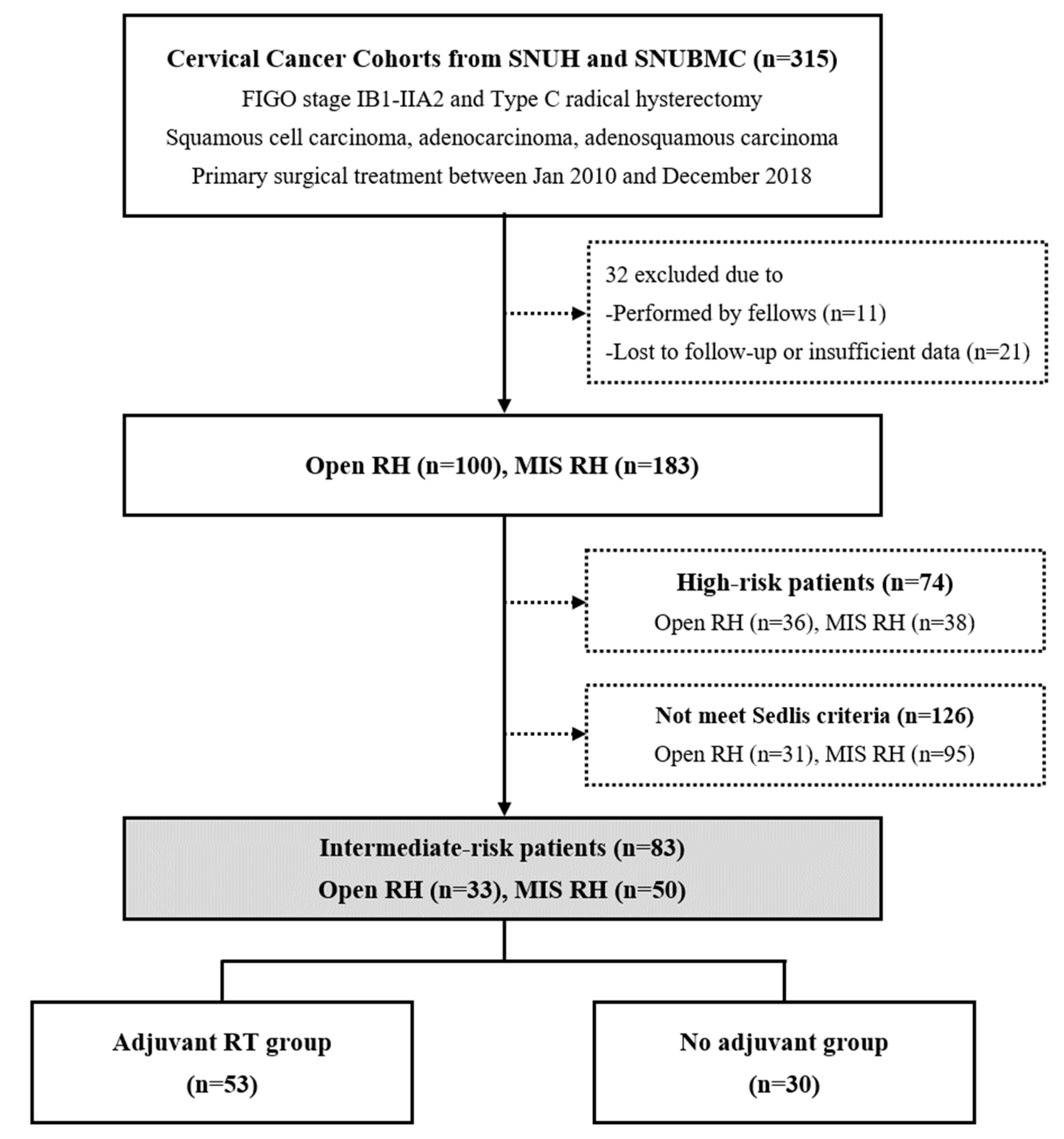
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Analysis in All Patients
3.2. Subgroup Analyses by Surgical Approach
3.3. Recurrence Patterns and Final Status of Recurred Patients
3.4. Guideline Adherence Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Won, Y.J.; Park, Y.R.; Jung, K.W.; Kong, H.J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat. 2020, 52, 335–350. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Cervical Cancer (Version 2.2020). Available online: http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 15 August 2020).
- Lim, M.C.; Lee, M.; Shim, S.H.; Nam, E.J.; Lee, J.Y.; Kim, H.J.; Lee, Y.Y.; Lee, K.B.; Park, J.Y.; Kim, Y.H.; et al. Practice guidelines for management of cervical cancer in Korea: A Korean Society of Gynecologic Oncology Consensus Statement. J. Gynecol. Oncol. 2017, 28, e22. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Doo, D.W.; Kirkland, C.T.; Griswold, L.H.; McGwin, G.; Huh, W.K.; Leath, C.A., 3rd; Kim, K.H. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: Results from a single high volume institution. Gynecol. Oncol. 2019, 153, 242–247. [Google Scholar] [CrossRef]
- Kim, S.I.; Cho, J.H.; Seol, A.; Kim, Y.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol. Oncol. 2019, 153, 3–12. [Google Scholar] [CrossRef]
- Uppal, S.; Gehrig, P.A.; Peng, K.; Bixel, K.L.; Matsuo, K.; Vetter, M.H.; Davidson, B.A.; Cisa, M.P.; Lees, B.F.; Brunette, L.L.; et al. Recurrence Rates in Patients With Cervical Cancer Treated With Abdominal Versus Minimally Invasive Radical Hysterectomy: A Multi-Institutional Retrospective Review Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1030–1040. [Google Scholar] [CrossRef]
- Paik, E.S.; Lim, M.C.; Kim, M.H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.M.; Lee, C.; Choi, C.H.; et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: Ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol. Oncol. 2019, 154, 547–553. [Google Scholar] [CrossRef] [PubMed]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.Y.; Kim, M.H.; Nam, B.H.; Lee, T.S.; Song, E.S.; Park, C.Y.; Kim, J.W.; Kim, Y.B.; Ryu, H.S.; Park, S.Y.; et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: A Korean Gynecologic Oncology Group study. Br. J. Cancer 2014, 110, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Cibula, D.; Abu-Rustum, N.R.; Fischerova, D.; Pather, S.; Lavigne, K.; Slama, J.; Alektiar, K.; Ming-Yin, L.; Kocian, R.; Germanova, A.; et al. Surgical treatment of “intermediate risk” lymph node negative cervical cancer patients without adjuvant radiotherapy-A retrospective cohort study and review of the literature. Gynecol. Oncol. 2018, 151, 438–443. [Google Scholar] [CrossRef]
- Van der Velden, J.; Mom, C.H.; van Lonkhuijzen, L.; Tjiong, M.Y.; Westerveld, H.; Fons, G. Analysis of isolated loco-regional recurrence rate in intermediate risk early cervical cancer after a type C2 radical hysterectomy without adjuvant radiotherapy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019. [Google Scholar] [CrossRef]
- Tewari, K.S. Minimally Invasive Surgery for Early-Stage Cervical Carcinoma: Interpreting the Laparoscopic Approach to Cervical Cancer Trial Results. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 3075–3080. [Google Scholar] [CrossRef]
- Kong, T.W.; Chang, S.J.; Piao, X.; Paek, J.; Lee, Y.; Lee, E.J.; Chun, M.; Ryu, H.S. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J. Obstet. Gynaecol. Res. 2016, 42, 77–86. [Google Scholar] [CrossRef]
- Kim, H.; Park, W.; Kim, Y.S.; Kim, Y.J. Chemoradiotherapy is not superior to radiotherapy alone after radical surgery for cervical cancer patients with intermediate-risk factor. J. Gynecol. Oncol. 2020, 31, e35. [Google Scholar] [CrossRef] [Green Version]


| Variables | Adjuvant Radiotherapy (n = 53, %) | No Adjuvant Treatment (n = 30, %) | p |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 51.6 ± 11.5 | 53.2 ± 14.2 | 0.587 |
| Surgical approach | 0.776 | ||
| Open | 22 (41.5) | 11 (36.7) | |
| Laparoscopy | 28 (52.8) | 18 (60.0) | |
| Robot-assisted surgery | 3 (5.7) | 1 (3.3) | |
| Conization | 9 (17.0) | 10 (33.3) | 0.088 |
| Histologic type | 0.006 | ||
| Squamous cell carcinoma | 48 (90.6) | 21 (70.0) | |
| Adenocarcinoma | 1 (1.9) | 7 (23.3) | |
| Adenosquamous carcinoma | 4 (7.5) | 2 (6.7) | |
| 2009 FIGO stage | 0.375 | ||
| IB1 | 28 (52.8) | 20 (66.7) | |
| IB2 | 13 (24.5) | 7 (23.3) | |
| IIA1 | 4 (7.5) | 2 (6.7) | |
| IIA2 | 8 (15.1) | 1 (3.3) | |
| Para-aortic LN sampling/dissection | 11 (20.8) | 7 (23.3) | 0.784 |
| Clinical cervical tumor size, mm | |||
| Mean ± SD | 34.8 ± 17.0 | 25.7 ± 17.5 | 0.024 |
| Pathologic cervical tumor size, mm | |||
| Mean ± SD | 50.5 ± 18.1 | 45.8 ± 12.6 | 0.219 |
| <20 | 1 (1.9) | 1 (3.3) | 0.976 |
| ≥20 and <40 | 14 (26.4) | 8 (26.7) | |
| ≥40 and <50 | 10 (18.9) | 6 (20.0) | |
| ≥50 | 28 (52.8) | 15 (50.0) | |
| LVSI | 35 (66.0) | 15 (50.0) | 0.151 |
| Stromal invasion | 0.001 | ||
| Superficial 1/3 | 1 (1.9) | 1 (3.3) | |
| Middle 1/3 | 4 (7.5) | 12 (40.0) | |
| Deep 1/3 | 48 (90.6) | 17 (56.7) | |
| Sedlis criteria | 0.034 | ||
| LVSI (+) Deep 1/3, Tumor size any | 33 (62.3) | 10 (33.3) | |
| LVSI(+) Middle 1/3, Tumor ≥ 20 mm | 1 (1.9) | 4 (13.3) | |
| LVSI(+) Superficial 1/3, Tumor ≥ 50 mm | 1 (1.9) | 1 (3.3) | |
| LVSI(-), Middle or deep 1/3, Tumor ≥ 40 mm | 18 (34.0) | 15 (50.0) | |
| Adjuvant treatment | N/A | ||
| RT only | 11 (20.8) | 0 | |
| CCRT | 42 (79.2) | 0 |
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | aHR | 95% CI | p | ||
| Age, years | ≥50 vs. <50 | 0.558 | 0.198–1.573 | 0.270 | 0.427 | 0.144–1.270 | 0.126 |
| Pre-operative conization | Yes vs. No | 0.867 | 0.244–3.076 | 0.825 | |||
| Histologic type | Non-SCC vs. SCC | 0.826 | 0.186–3.679 | 0.802 | 0.453 | 0.096–2.137 | 0.317 |
| 2009 FIGO stage | IB2-IIA2 vs. IB1 | 0.715 | 0.244–2.093 | 0.541 | 0.738 | 0.236–2.301 | 0.600 |
| Cervical tumor size, mm | Pathologic, ≥40 vs. <40 | 0.896 | 0.306–2.623 | 0.841 | |||
| Deep stromal invasion | Yes vs. No | 0.416 | 0.141–1.221 | 0.110 | |||
| Surgical approach | MIS vs. Open | 1.868 | 0.594–5.874 | 0.285 | 1.730 | 0.532–5.626 | 0.362 |
| Adjuvant treatment | RT vs. No adjuvant | 0.268 | 0.094–0.766 | 0.014 | 0.241 | 0.082–0.709 | 0.010 |
| Variables | Open RH (n = 33) | MIS RH (n = 50) | |||||
|---|---|---|---|---|---|---|---|
| aHR | 95% CI | p | aHR | 95% CI | p | ||
| Age, years | ≥50 vs. <50 | 0.176 | 0.015–2.060 | 0.166 | 0.621 | 0.178–2.166 | 0.455 |
| Histologic type | Non-SCC vs. SCC | 0.851 | 0.171–4.228 | 0.844 | |||
| 2009 FIGO stage | IB2-IIA2 vs. IB1 | 0.377 | 0.033–4.346 | 0.434 | |||
| Cervical tumor size, mm | Clinical, ≥40 vs. <40 | 1.648 | 0.354–7.681 | 0.525 | |||
| Deep stromal invasion | Yes vs. No | 0.102 | 0.004–2.954 | 0.184 | |||
| Adjuvant treatment | RT vs. No adjuvant | 0.098 | 0.009–1.027 | 0.053 | 0.305 | 0.077–1.214 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.I.; Kim, T.H.; Lee, M.; Kim, H.S.; Chung, H.H.; Lee, T.S.; Jeon, H.W.; Kim, J.-W.; Park, N.H.; Song, Y.S. Impact of Adjuvant Radiotherapy on Survival Outcomes in Intermediate-Risk, Early-Stage Cervical Cancer: Analyses Regarding Surgical Approach of Radical Hysterectomy. J. Clin. Med. 2020, 9, 3545. https://doi.org/10.3390/jcm9113545
Kim SI, Kim TH, Lee M, Kim HS, Chung HH, Lee TS, Jeon HW, Kim J-W, Park NH, Song YS. Impact of Adjuvant Radiotherapy on Survival Outcomes in Intermediate-Risk, Early-Stage Cervical Cancer: Analyses Regarding Surgical Approach of Radical Hysterectomy. Journal of Clinical Medicine. 2020; 9(11):3545. https://doi.org/10.3390/jcm9113545
Chicago/Turabian StyleKim, Se Ik, Tae Hun Kim, Maria Lee, Hee Seung Kim, Hyun Hoon Chung, Taek Sang Lee, Hye Won Jeon, Jae-Weon Kim, Noh Hyun Park, and Yong Sang Song. 2020. "Impact of Adjuvant Radiotherapy on Survival Outcomes in Intermediate-Risk, Early-Stage Cervical Cancer: Analyses Regarding Surgical Approach of Radical Hysterectomy" Journal of Clinical Medicine 9, no. 11: 3545. https://doi.org/10.3390/jcm9113545






