Serum Levels of T Cell Immunoglobulin and Mucin-Domain Containing Molecule 3 in Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Evaluations
2.2. Enzyme-Linked Immunosorbent Assay for Soluble TIM-3
2.3. Statistical Analysis
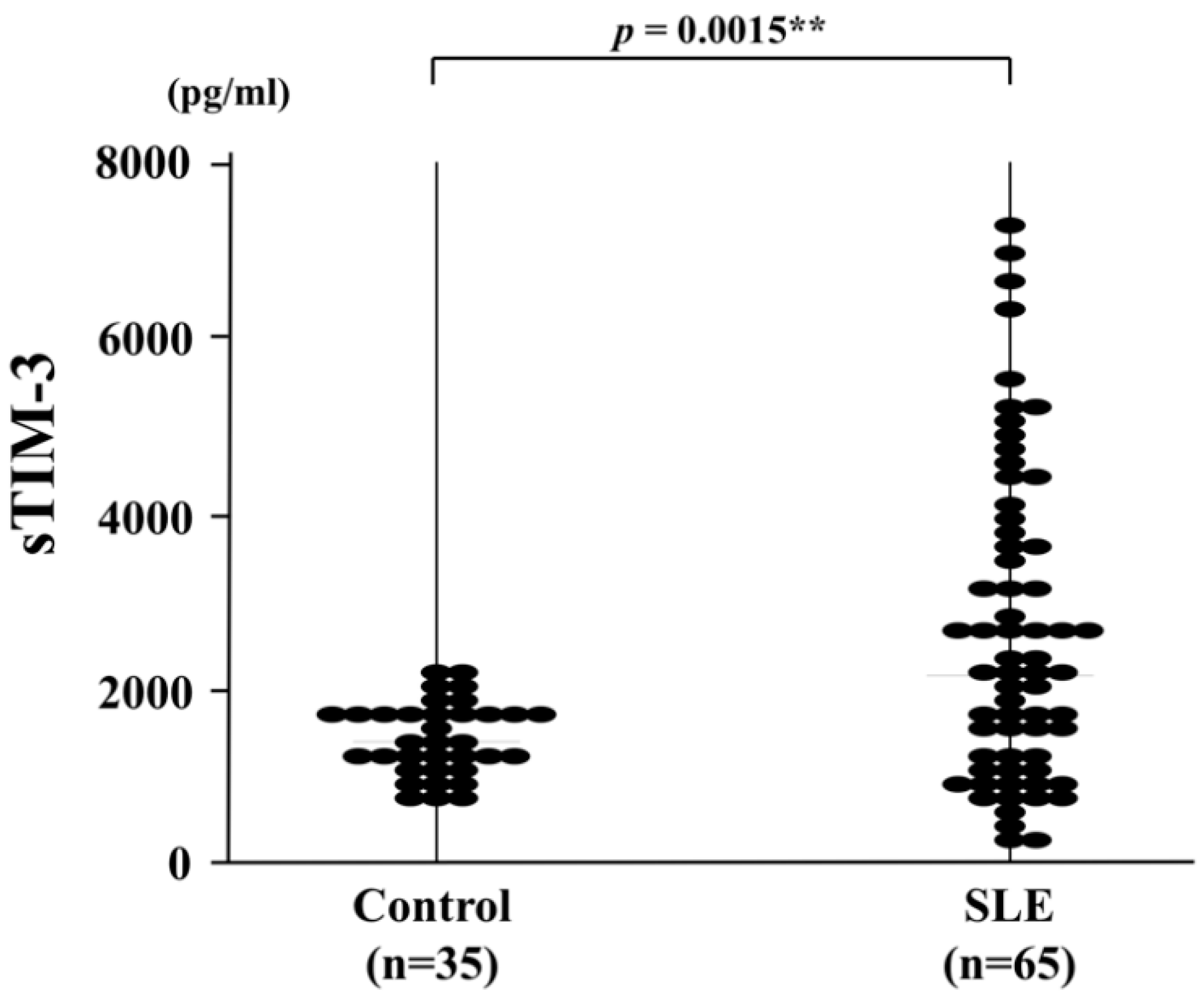
3. Results
3.1. Demographic and Clinical Characteristics of SLE Patients
3.2. Correlation between Serum sTIM-3 Levels and Disease Activity of SLE
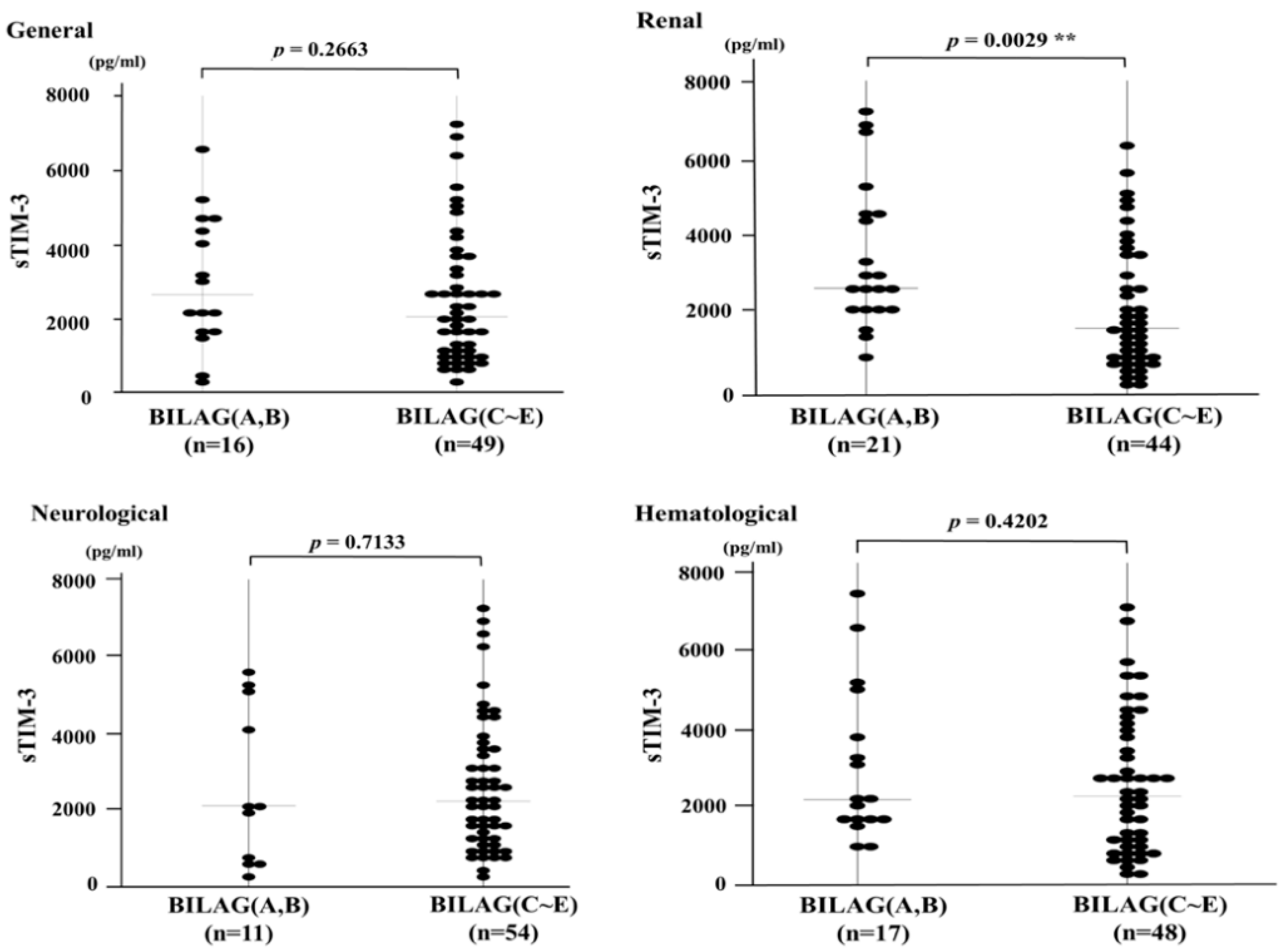
3.3. Serum Levels of sTIM-3 and Organ Involvement Measured by the BILAG-2004 Index
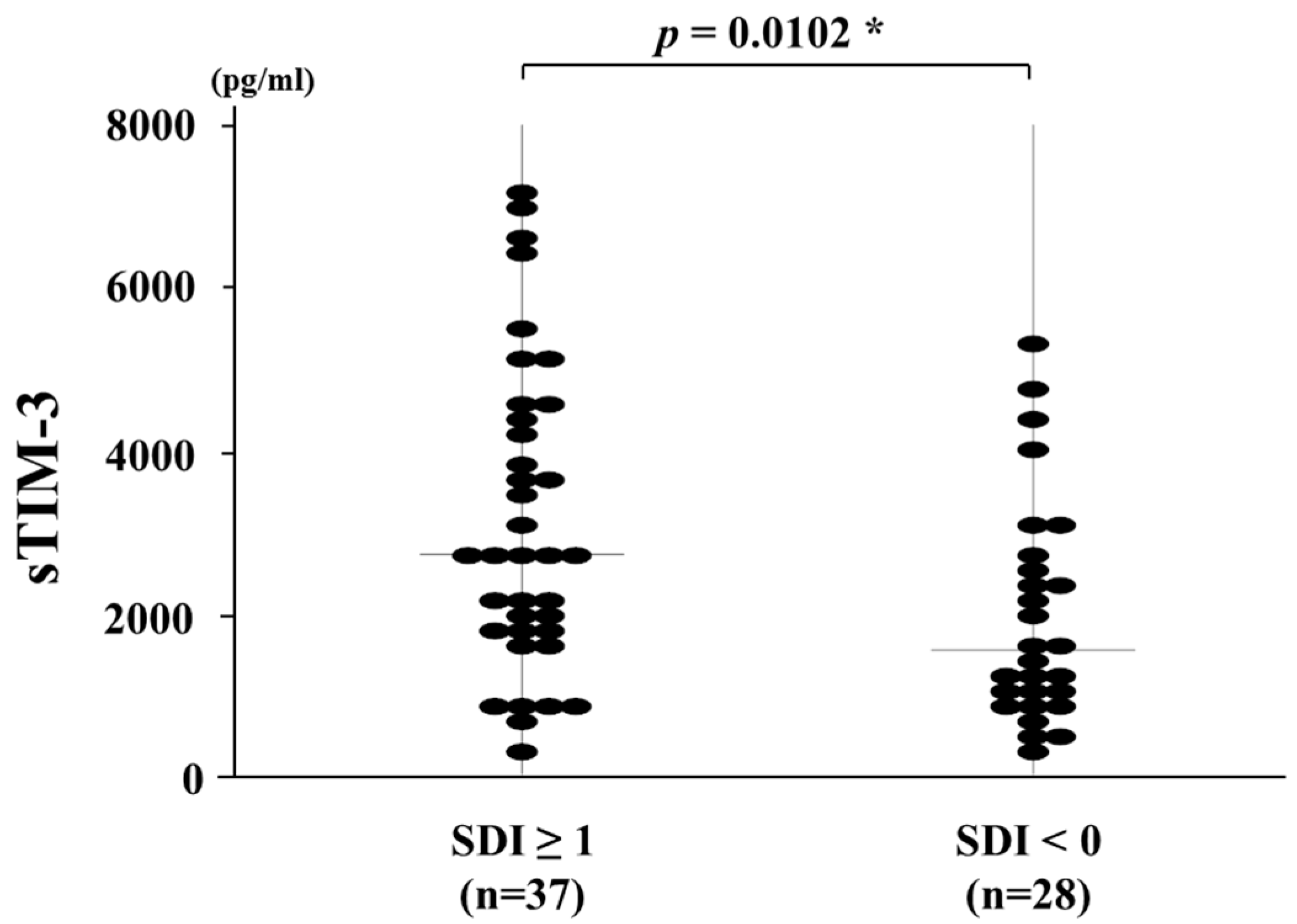
3.4. Circulating Levels of sTIM-3 and Organ Damage Measured by the SDI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | a disintegrin and metalloproteinase with a thrombospondin type 1 motif |
| BILAG-2004 | British Isles Lupus Assessment Group 2004 |
| IQR | Interquartile range |
| Gal-9 | Galectin-9 |
| SLE | Systemic lupus erythematosus |
| SLEDAI | Systemic Lupus Erythematosus Disease Activity Index |
| SLICC | Systemic Lupus International Collaborating Clinics |
| TIM-3 | T cell immunoglobulin and mucin-domain containing 3 |
References
- Zhang, Q.; Vignali, D.A. Co-stimulatory and Co-inhibitory pathways in autoimmunity. Immunity 2016, 44, 1034–1051. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Anderson, D.E. TIM-3 in autoimmunity. Curr. Opin. Immunol. 2006, 18, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fueyo, A.; Tian, J.; Picarella, D.; Domenig, C.; Zheng, X.X.; Sabatos, C.A.; Manlongat, N.; Bender, O.; Kamradt, T.; Kuchroo, V.K.; et al. Tim-3 inhibits T helper type 1-mediated auto—and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003, 4, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.F.; Zhang, N.; Li, W.X.; Tao, J.H.; Ye, D.Q. TIM-3 as a new therapeutic target in systemic lupus erythematosus. Mol. Biol. Rep. 2010, 37, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, T.; Tsokos, G.C.; Moulton, V.R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 2018, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Ni, L.; Dong, C. New checkpoints in cancer immunotherapy. Immunol. Rev. 2017, 276, 52–65. [Google Scholar] [CrossRef]
- Sebastiani, G.D.; Scirocco, C.; Galeazzi, M. Rheumatic immune related adverse events in patients treated with checkpoint inhibitors for immunotherapy of cancer. Autoimmun. Rev. 2019, 18, 805–813. [Google Scholar] [CrossRef]
- Boenisch, O.; D’Addio, F.; Watanabe, T.; Elyaman, W.; Magee, C.N.; Yeung, M.Y.; Padera, R.F.; Rodig, S.J.; Murayama, T.; Tanaka, K.; et al. TIM-3: A novel regulatory molecule of alloimmune activation. J. Immunol. 2010, 185, 5806–5819. [Google Scholar] [CrossRef]
- Van den Hoogen, L.L.; van Roon, J.A.G.; Mertens, J.S.; Wienke, J.; Lopes, A.P.; de Jager, W.; Rossato, M.; Pandit, A.; Wichers, C.G.K.; van Wijk, F.; et al. Galectin-9 is an easy to measure biomarker for the interferon signature in systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2018, 77, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Jayaraman, P.; Behar, S.M.; Anderson, A.C.; Kuchroo, V.K. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011, 32, 345–349. [Google Scholar] [CrossRef]
- Song, L.J.; Wang, X.; Wang, X.P.; Li, D.; Ding, F.; Liu, H.X.; Yu, X.; Li, X.F.; Shu, Q. Increased Tim-3 expression on peripheral T lymphocyte subsets and association with higher disease activity in systemic lupus erythematosus. Diagn. Pathol. 2015, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Möller-Hackbarth, K.; Dewitz, C.; Schweigert, O.; Trad, A.; Garbers, C.; Rose-John, S.; Scheller, J. A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3). J. Biol. Chem. 2013, 288, 34529–34544. [Google Scholar] [CrossRef]
- Chiba, M.; Yanaba, K.; Hayashi, M.; Yoshihara, Y.; Nakagawa, H. Clinical significance of serum soluble T-cell immunoglobulin and mucin domain 3 levels in systemic sclerosis: Association with disease severity. J. Derm. 2017, 44, 194–197. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; Hay, E.; et al. The development and initial validation of the systemic lupus international collaborating clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996, 39, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, D.A.; Rahman, A.; Allen, E.; Farewell, V.; Akil, M.; Bruce, I.N.; D’Cruz, D.; Griffiths, B.; Khamashta, M.; Maddison, P.; et al. BILAG 2004. Development and initial validation of an updated version of the british isles lupus assessment group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005, 44, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Stoll, T.; Stucki, G.; Malik, J.; Pyke, S.; Isenberg, D.A. Further validation of the BILAG disease activity index in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 1996, 55, 756–760. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Kuchroo, V.K.; Umetsu, D.T.; DeKruyff, R.H.; Freeman, G.J. The TIM gene family: Emerging roles in immunity and disease. Nat. Rev. Immunol. 2003, 3, 454–462. [Google Scholar] [CrossRef]
- Moulton, V.R.; Suarez-Fueyo, A.; Meidan, E.; Li, H.; Mizui, M.; Tsokos, G.C. Pathogenesis of human systemic lupus erythematosus: A cellular perspective. Trends Mol. Med. 2017, 23, 615–635. [Google Scholar] [CrossRef]
- Rao, V.; Gordon, C. Advances in the assessment of lupus disease activity and damage. Curr. Opin. Rheumatol. 2014, 26, 510–519. [Google Scholar] [CrossRef]
- Jiao, Q.; Qian, Q.; Zhao, Z.; Fang, F.; Hu, X.; An, J.; Wu, J.; Liu, C. Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) and TIM-3 ligands in peripheral blood from patients with systemic lupus erythematosus. Arch. Derm. Res. 2016, 308, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, N.; Fujita, Y.; Temmoku, J.; Furuya, M.Y.; Asano, T.; Sato, S.; Matsumoto, H.; Kobayashi, H.; Watanabe, H.; Suzuki, E.; et al. Galectin-9 as a biomarker for disease activity in systemic lupus erythematosus. PLoS ONE 2020, 15, e0227069. [Google Scholar] [CrossRef] [PubMed]
- Moritoki, M.; Kadowaki, T.; Niki, T.; Nakano, D.; Soma, G.; Mori, H.; Kobara, H.; Masaki, T.; Kohno, M.; Hirashima, M. Galectin-9 ameliorates clinical severity of MRL/lpr lupus-prone mice by inducing plasma cell apoptosis independently of Tim-3. PLoS ONE 2013, 8, e60807. [Google Scholar] [CrossRef]
- Geng, H.; Zhang, G.M.; Li, D.; Zhang, H.; Yuan, Y.; Zhu, H.G.; Xiao, H.; Han, L.F.; Feng, Z.H. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J. Immunol. 2006, 176, 1411–1420. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R.; Zhou, J.; Shi, W.; Xu, L.; Sheng, J.; Peng, H.; Jin, Y.; Yuan, H. Association of serum T cell immunoglobulin domain and Mucin-3 and Interleukin-17 with systemic lupus erythematosus. Med. Sci. Monit. Basic Res. 2018, 24, 168–176. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 65 |
|---|---|
| Sex | |
| Female, n (%) | 56 (84) |
| Male, n (%) | 11 (16) |
| Median age (range), years | 33 (16–79) |
| Median disease duration of SLE (range), months | 55 (1–420) |
| Untreated patients, n (%) | 55 (83) |
| Items of SLE classification criteria, n (%) | |
| Rash | 32 (48) |
| Alopecia | 3 (4) |
| Oral ulcer | 5 (7) |
| Arthritis | 22 (33) |
| Serositis | 17 (25) |
| Renal disorder | 38 (57) |
| Laboratory findings, n (%) | |
| Leukocytopenia | 25 (37) |
| Thrombocytopenia | 18 (27) |
| Anti-double stranded-DNA antibody positive | 52 (78) |
| Anti-smith antibody positive | 31 (46) |
| Anti-phospholipid antibody positive | 28 (42) |
| Median SLEDAI, index (range) | 14 (0–50) |
| Median SDI, index (range) | 1 (0–4) |
| BILAG | Grade (n = 65) | ||||
|---|---|---|---|---|---|
| Manifestations | A | B | C | D | E |
| General | 3 | 14 | 16 | 4 | 29 |
| Mucocutaneous | 1 | 5 | 19 | 6 | 35 |
| Neurological | 2 | 10 | 1 | 1 | 52 |
| Musculoskeletal | 2 | 4 | 14 | 4 | 42 |
| Cardiovascular/respiratory | 0 | 6 | 9 | 3 | 48 |
| Abdominal | 1 | 7 | 0 | 1 | 57 |
| Renal | 15 | 6 | 6 | 5 | 34 |
| Hematological | 2 | 15 | 36 | 3 | 10 |
| Ophthalmic | 1 | 4 | 6 | 2 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, T.; Matsuoka, N.; Fujita, Y.; Matsumoto, H.; Temmoku, J.; Yashiro-Furuya, M.; Sato, S.; Suzuki, E.; Kobayashi, H.; Watanabe, H.; et al. Serum Levels of T Cell Immunoglobulin and Mucin-Domain Containing Molecule 3 in Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2020, 9, 3563. https://doi.org/10.3390/jcm9113563
Asano T, Matsuoka N, Fujita Y, Matsumoto H, Temmoku J, Yashiro-Furuya M, Sato S, Suzuki E, Kobayashi H, Watanabe H, et al. Serum Levels of T Cell Immunoglobulin and Mucin-Domain Containing Molecule 3 in Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine. 2020; 9(11):3563. https://doi.org/10.3390/jcm9113563
Chicago/Turabian StyleAsano, Tomoyuki, Naoki Matsuoka, Yuya Fujita, Haruki Matsumoto, Jumpei Temmoku, Makiko Yashiro-Furuya, Shuzo Sato, Eiji Suzuki, Hiroko Kobayashi, Hiroshi Watanabe, and et al. 2020. "Serum Levels of T Cell Immunoglobulin and Mucin-Domain Containing Molecule 3 in Patients with Systemic Lupus Erythematosus" Journal of Clinical Medicine 9, no. 11: 3563. https://doi.org/10.3390/jcm9113563
APA StyleAsano, T., Matsuoka, N., Fujita, Y., Matsumoto, H., Temmoku, J., Yashiro-Furuya, M., Sato, S., Suzuki, E., Kobayashi, H., Watanabe, H., & Migita, K. (2020). Serum Levels of T Cell Immunoglobulin and Mucin-Domain Containing Molecule 3 in Patients with Systemic Lupus Erythematosus. Journal of Clinical Medicine, 9(11), 3563. https://doi.org/10.3390/jcm9113563





