Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta
Abstract
1. Introduction
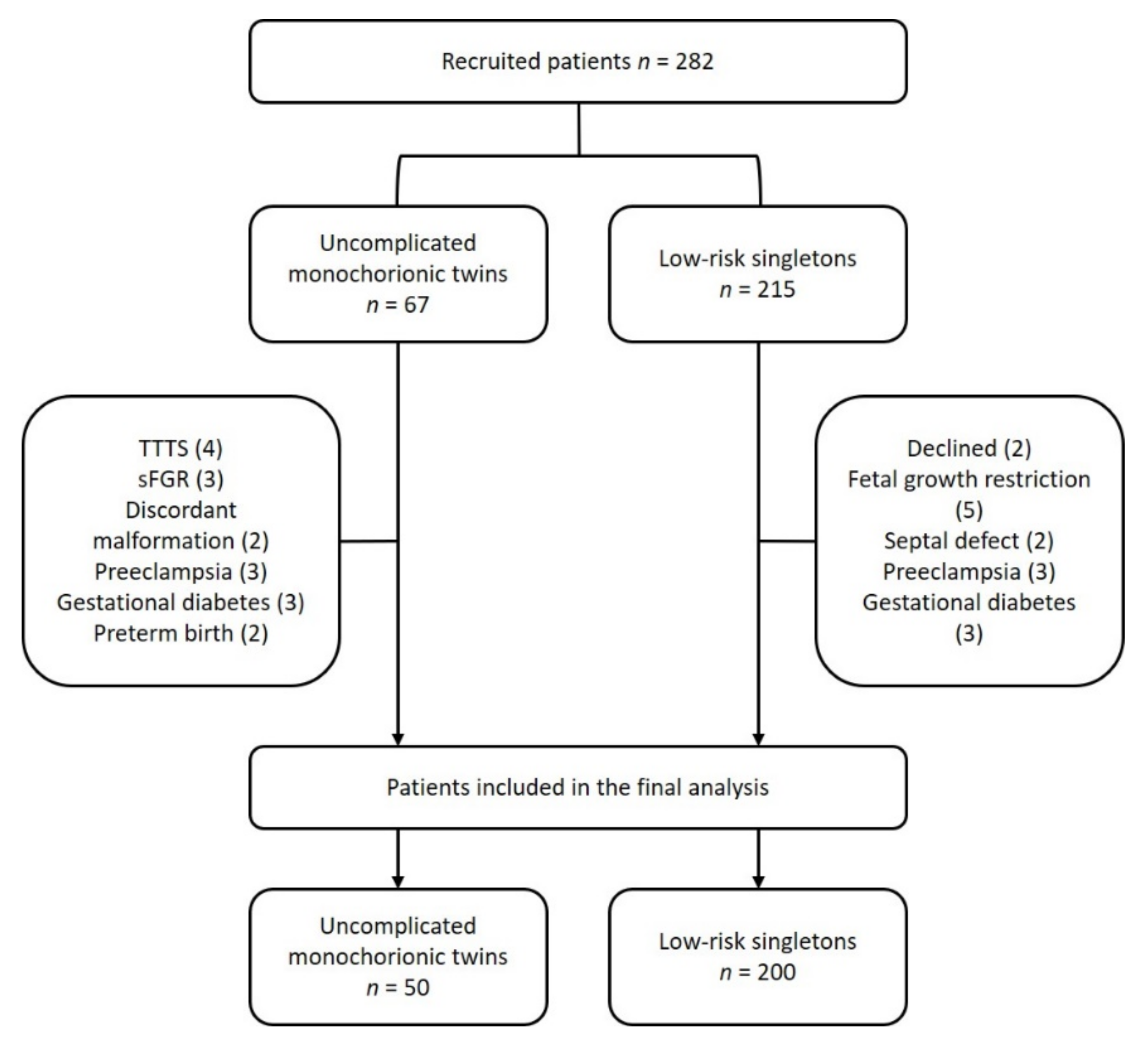
2. Materials and Methods
2.1. Study Protocol
2.2. Fetal Ultrasound Evaluation
2.3. Cord Blood Sampling and B-Type Natriuretic Peptide Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline and Perinatal Data
3.2. Echocardiography
3.3. Echocardiography z-Scores
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denbow, M.L.; Cox, P.; Taylor, M.; Hammal, D.M.; Fisk, N.M. Placental angioarchitecture in monochorionic twin pregnancies: Relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am. J. Obstet. Gynecol. 2000, 182, 417–426. [Google Scholar] [CrossRef]
- Lewi, L.; Deprest, J.; Hecher, K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am. J. Obstet. Gynecol. 2013, 208, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Camm, E.J.; Botting, K.J.; Sferruzzi-Perri, A.N. Near to One’s Heart: The Intimate Relationship Between the Placenta and Fetal Heart. Front. Physiol. 2018, 26, 629. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E.; Burton, G.J. Development of the Human Placenta and Fetal Heart: Synergic or Independent? Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Gardiner, H.M.; Matsui, H.; Roughton, M.; Greenwald, S.E.; Diemert, A.; Taylor, M.J.O.; Hecher, K. Cardiac function in 10-year-old twins following different fetal therapies for twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2014, 43, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, H.M. Response of the fetal heart to changes in load: From hyperplasia to heart failure. Heart 2005, 91, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017, 2, 1012–1026. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Boudreaux, D.; Moise, K.J.; Johnson, A.; Papanna, R.; Bebbington, M.; Gardiner, H.M. Cardiac pathophysiology in twin–twin transfusion syndrome: New insights into its evolution. Ultrasound Obstet. Gynecol. 2018, 51, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Abellana, B.; Hernandez-Andrade, E.; Figueroa-Diesel, H.; Ferrer, Q.; Acosta-Rojas, R.; Roura, L.C.; Gratacós, E. Hypertrophic cardiomyopathy-like changes in monochorionic twin pregnancies with selective intrauterine growth restriction and intermittent absent/reversed end-diastolic flow in the umbilical artery. Ultrasound Obstet. Gynecol. 2007, 30, 977–982. [Google Scholar] [CrossRef]
- Society for Maternal-fetal Medicine (SMFM); Simpson, L.L. SMFM Clinical Guideline: Twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2018, 208, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Gratacós, E.; Lewi, L.; Muñoz, B.; Acosta-Rojas, R.; Hernandez-Andrade, E.; Martinez, J.M.; Carreras, E.; Deprest, J. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet. Gynecol. 2007, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.U.; Torres, X.; Eixarch, E.; Bennasar, M.; Cruz-Lemini, M.; Gomez, O.; Lobmaier, S.M.; Martinez, J.M.; Gratacós, E.; Crispi, F. Differential changes in myocardial performance index and its time intervals in donors and recipients of twin-to-twin transfusion syndrome before and after laser therapy. Fetal Diagn. Ther. 2018, 44. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.U.; Crispi, F.; Yamamoto, R.; Masoller, N.; Luewan, S.; Gomez, O.; Bennasar, M.; Lobmaier, S.M.; Eixarch, E.; Martinez, J.M.; et al. Longitudinal annular displacement by M-mode (MAPSE and TAPSE) in twin-to-twin transfusion syndrome before and after laser surgery. Prenat Diagn. 2015, 35, 1197–1201. [Google Scholar] [CrossRef]
- Fujioka, K.; Mizobuchi, M.; Sakai, H.; Iwatani, S.; Wada, K.; Yoshimoto, S.; Nakao, H. N-terminal pro-brain natriuretic peptide levels in monochorionic diamniotic twins with selective intrauterine growth restriction. J. Perinatol. 2014, 6–10. [Google Scholar] [CrossRef]
- Fujioka, K.; Sakai, H.; Tanaka, S.; Iwatani, S.; Wada, K.; Mizobuchi, M.; Yoshimoto, S.; Nakao, H. N-terminal Pro-brain Natriuretic Peptide Levels in Monochorionic Diamniotic Twins with Twin-to-twin Transfusion Syndrome Treated by Fetoscopic Laser Photocoagulation. Kobe J. Med. Sci. 2013, 59, 28–35. [Google Scholar]
- Robinson, H.P. Sonar Measurement of Fetal Crown- Rump Length as Means of Assessing Maturity in First Trimester of Pregnancy. Br. Med. J. 1973, 4, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.; Oepkes, D.; et al. ISUOG Practice Guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef]
- Hadlock, F.; Harrist, R.; Carpenter, R.; Deter, R.; Park, S. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984, 150, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J. Customized birthweight standards for a Spanish population. Eur. J. Obs. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef]
- Torres, X.; Bennasar, M.; Eixarch, E.; Rueda, C.; Goncé, A.; Muñoz, M.; Marimon, E.; Martínez, J.M.; Gratacós, E.; Figueras, F. Gender-Specific Antenatal Growth Reference Charts in Monochorionic Twins. Fetal Diagn. Ther. 2018, 44. [Google Scholar] [CrossRef]
- Winn, H.N.; Gabrielli, S.; Reece, E.A.; Roberts, J.A.; Salafia, C.; Hobbins, J.C. Ultrasonographic criteria for the prenatal diagnosis of placental chorionicity in twin gestations. Am. J. Obstet. Gynecol. 1989, 161, 1540–1542. [Google Scholar] [CrossRef]
- Arduini, D.; Rizzo, G. Normal values of Pulsatility Index from fetal vessels: A cross-sectional study on 1556 healthy fetuses. J. Perinat Med. 1990, 18, 165–172. [Google Scholar] [CrossRef]
- Hecher, K.; Campbell, S.; Snijders, R.; Nicolaides, K. Reference ranges for fetal venous and atrioventricular blood flow parameters. Ultrasound Obstet. Gynecol. 1994, 4, 381–390. [Google Scholar] [CrossRef]
- Ciobanu, A.; Wright, A.; Syngelaki, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef]
- Kessler, J.; Rasmussen, S.; Hanson, M.; Kiserud, T. Longitudinal reference ranges for ductus venosus flow velocities and waveform indices. Ultrasound Obstet. Gynecol. 2006, 28, 890–898. [Google Scholar] [CrossRef]
- García-Otero, L.; Gomez, O.; Rodriguez-Lopez, M.; Torres, X.; Soveral, I.; Sepúlveda-Martínez, A.; Guirado, L.; Valenzuela-Alcaraz, B.; López, M.; Martínez, J.M.; et al. Nomograms of Fetal Cardiac Dimensions at 18-41 Weeks of Gestation. Fetal Diagn. Ther. 2018, 1–12. [Google Scholar] [CrossRef]
- García-Otero, L.; Soveral, I.; Sepúlveda-Martínez, Á.; Rodriguez-López, M.; Torres, X.; Guirado, L.; Nogué, L.; Valenzuela-Alcaraz, B.; Martínez, J.M.; Gratacós, E.; et al. Reference ranges for fetal cardiac, ventricular and atrial relative size, sphericity, ventricular dominance, wall asymmetry and relative wall thickness from 18 to 41 weeks of gestation. Ultrasound Obs. Gynecol. 2020. [Google Scholar] [CrossRef]
- Foppa, M.; Duncan, B.B.; Rohde, L.E.P. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc. Ultrasound 2005, 3, 1–13. [Google Scholar] [CrossRef]
- DeVore, G.R. Assessing fetal cardiac ventricular function. Semin. Fetal Neonatal. Med. 2005, 10, 515–541. [Google Scholar] [CrossRef]
- Cruz-Martinez, R.; Figueras, F.; Bennasar, M.; García-Posadas, R.; Crispi, F.; Hernandez-Andrade, E.; Gratacós, E. Normal reference ranges from 11 to 41 weeks’ gestation of fetal left modified myocardial performance index by conventional doppler with the use of stringent criteria for delimitation of the time periods. Fetal Diagn. Ther. 2012, 32, 79–86. [Google Scholar] [CrossRef]
- Zhao, Y.M.B.; Wang, B. Z-Score Reference Ranges for Angular M-Mode Displacement at 22–40 Weeks’ gestation. Fetal Diagn. Ther. 2017, 41, 115–126. [Google Scholar] [CrossRef]
- Hsieh, Y.; Chang, F.C.; Tsai, H. Longitudinal survey of fetal ventricular ejection and shortening fraction throughout pregnancy. Ultrasound Obs. Gynecol. 2000, 16, 46–48. [Google Scholar] [CrossRef]
- Kiserud, T.; Ebbing, C.; Kessler, J.; Rasmussen, S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obs. Gynecol. 2006, 28, 126–136. [Google Scholar] [CrossRef]
- Rasbash, J.; Browne, W.; Goldstein, H. Centre for Multilevel Modelling U of B. A user’s Guide to MLwiN v 2.36 n.d. Available online: http://www.bristol.ac.uk/cmm/software/mlwin (accessed on 23 October 2017).
- Gagnon, C.; Bigras, J.L.; Fouron, J.C.; Dallaire, F. Reference Values and Z Scores for Pulsed-Wave Doppler and M-Mode Measurements in Fetal Echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 448–460.e9. [Google Scholar] [CrossRef]
- Finneran, M.M.; Pickens, R.; Templin, M.; Stephenson, C.D. Impact of recipient twin preoperative myocardial performance index in twin–twin transfusion syndrome treated with laser. J. Matern. Neonatal. Med. 2017, 30, 767–771. [Google Scholar] [CrossRef]
- Zanardini, C.; Fichera, A.; Calza, S.; Cappa, V.; Orabona, R.; Frusca, T.; Prefumo, F. Longitudinal reference ranges for serial measurements of myocardial performance index (MPI) by conventional and pulsed-wave tissue Doppler in monochorionic diamniotic twins at 17 to 26 weeks of gestation. Prenat. Diagn. 2018, 38, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sueters, M.; Middeldorp, J.M.; Lopriore, E.; Bökenkamp, R.; Oepkes, D.; Teunissen, K.A.; Kanhai, H.H.H.; Le Cessie, S.; Vandenbussche, F.P.H.A. Fetal cardiac output in monochorionic twins. Ultrasound Obstet. Gynecol. 2008, 32, 807–812. [Google Scholar] [CrossRef]
- Maiz, N.; Staboulidou, I.; Leal, A.M.; Minekawa, R.; Nicolaides, K.H. Ductus venosus Doppler at 11 to 13 weeks of gestation in the prediction of outcome in twin pregnancies. Obstet. Gynecol. 2009, 113, 860–865. [Google Scholar] [CrossRef]
- Zoppi, M.A. Nuchal translucency screening in monochorionic twin pregnancies. Ultrasound Obstet. Gynecol. 2009, 34, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Casati, D.; Pellegrino, M.; Cortinovis, I.; Spada, E.; Lanna, M.; Faiola, S.; Cetin, I.; Rustico, M.A. Longitudinal Doppler references for monochorionic twins and comparison with singletons. PLoS ONE 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Sueters, M.; Middeldorp, J.M.; Vandenbussche, F.P.H.A.; Teunissen, K.A.; Lopriore, E.; Kanhai, H.H.H.; Le Cessie, S.; Oepkes, D. The effect of fetoscopic laser therapy on fetal cardiac size in twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2008, 31, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Karatza, A.A.; Wolfenden, J.L.; Taylor, M.J.O.; Wee, L.; Fisk, N.M.; Gardiner, H.M. Influence of twin-twin transfusion syndrome on fetal cardiovascular structure and function: Prospective case–control study of 136 monochorionic twin pregnancies. Heart 2002, 88, 271–277. [Google Scholar] [CrossRef]
- Bajoria, R.; Ward, S.; Chatterjee, R. Brain natriuretic peptide and endothelin-1 in the pathogenesis of polyhydramnios-oligohydramnios in monochorionic twins. Am. J. Obstet. Gynecol. 2003, 189, 189–194. [Google Scholar] [CrossRef]
- Rodriguez, D.; Garcia-Rivas, G.; Laresgoiti-Servitje, E.; Yañez, J.; Torre-Amione, G.; Jerjes-Sanchez, C. B-type natriuretic peptide reference interval of newborns from healthy and pre-eclamptic women: A prospective, multicentre, cross-sectional study. BMJ Open 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Doust, J.A.; Pietrzak, E.; Dobson, A.; Glasziou, P.P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: Systematic review. Br. Med. J. 2005, 330, 625. [Google Scholar] [CrossRef]
- Van Mieghem, T.; Done, E.; Gucciardo, L.; Klaritsch, P.; Allegaert, K.; Van Bree, R.; Lewi, L.; Deprest, J. Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2010, 202, e1–e48. [Google Scholar] [CrossRef]
- Sebire, N.J.; Talbert, D.; Fisk, N.M. Twin-to-twin transfusion syndrome results from dynamic asymmetrical reduction in placental anastomoses: A hypothesis. Placenta 2001, 22, 383–391. [Google Scholar] [CrossRef]
- Katz, A.M. Ernest Henry Starling, his predecessors, and the “Law of the Heart”. Circulation 2002, 106, 2986–2992. [Google Scholar] [CrossRef]
- Sepulveda-Martinez, A.; Garcia-Otero, L.; Soveral, I.; Guirado, L.; Valenzuela-Alcaraz, B.; Torres, X.; Rodriguez-Lopez, M.; Gratacos, E.; Gomez, O.; Crispi, F. Comparison of 2D versus M-mode echocardiography for assessing fetal myocardial wall thickness. J. Matern Neonatal Med. 2019, 32. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | MCDA Fetuses (n = 100) | Singleton (n = 200) | p |
|---|---|---|---|
| Maternal | |||
| Age (years) | 33 (4) | 32 (4) | 0.406 |
| Height (m) | 1.63 (0.1) | 1.63 (0.1) | 0.225 |
| Weight (kg) | 60.9 (6.9) | 61.3 (7.4) | 0.079 |
| Body mass index (kg/m2) | 23.2 (2.5) | 23.8 (3.2) | 0.110 |
| Caucasian ethnicity | 83% | 81% | 0.105 |
| Smoking during pregnancy | 2% | 9% | 0.002 |
| Low socioeconomic status | 6% | 11% | 0.162 |
| Primiparous | 55% | 61% | 0.221 |
| Perinatal data | |||
| Gestational age at birth (weeks) | 36.3 (1.1) | 39.5 (1.6) | <0.001 |
| Birthweight (g) | 2395 (380) | 3255 (477) | <0.001 |
| Male sex | 42% | 50% | 0.192 |
| Cesarean section | 65% | 15% | <0.001 |
| Apgar at 5 min | 9 [7–10] | 9 [7–10] | 0.498 |
| Umbilical artery pH | 7.22 [7.16–7.28] | 7.21 [7.15–7.26] | 0.450 |
| Characteristic | MCDA Fetuses (n = 100) | Singleton (n = 200) | p * |
|---|---|---|---|
| Gestational age at ultrasound (weeks) | 28.0 (2.2) | 28.0 (2.3) | 0.862 |
| Standard fetoplacental data | |||
| Estimated fetal weight at ultrasound (g) | 1122 (240) | 1213 (262) | 0.027 |
| Estimated fetal weight centile | 49 (24) | 55 (28) | 0.059 |
| Umbilical artery PI | 1.10 (0.2) | 1.03 (0.2) | 0.191 |
| Middle cerebral artery PI | 1.84 (0.2) | 1.82 (0.3) | 0.743 |
| Ductus venosus PI | 0.60 (0.2) | 0.53 (0.1) | 0.007 |
| Heart rate | |||
| Left fetal heart rate (beats/min) | 140 (8) | 141 (7) | 0.092 |
| Right fetal heart rate (beats/min) | 141 (7) | 141 (7) | 0.102 |
| Cardiac morphometry | |||
| Aortic diameter (mm) | 4.7 (0.6) | 4.5 (0.7) | 0.201 |
| Main pulmonary diameter (mm) | 5.0 (0.7) | 4.8 (0.8) | 0.110 |
| Cardiac area (cm2) | 8.1 (1.7) | 8.0 (4.5) | 0.554 |
| Thorax area (cm2) | 30 (6) | 31 (6) | 0.217 |
| Cardiothoracic ratio | 0.26 (0.02) | 0.26 (0.03) | 0.923 |
| Right atrial area (cm2) | 1.36 (0.4) | 1.24 (0.4) | 0.001 |
| Right atria-to-heart ratio | 0.17 (0.02) | 0.15 (0.01) | 0.018 |
| Right ventricular area (cm2) | 1.74 (0.5) | 1.85 (0.4) | 0.001 |
| Right ventricle-to-heart ratio | 0.21 (0.04) | 0.24 (0.03) | 0.001 |
| Left atrial area (cm2) | 1.37 (0.3) | 1.21 (0.3) | 0.001 |
| Left atria-to-heart ratio | 0.17 (0.03) | 0.15 (0.02) | <0.001 |
| Left ventricular area (cm2) | 1.87 (0.5) | 1.99 (0.5) | 0.001 |
| Left ventricle-to-heart ratio | 0.24 (0.04) | 0.26 (0.04) | 0.019 |
| Right longitudinal diameter (mm) | 17.1 (2.0) | 17.6 (2.1) | 0.001 |
| Right basal transverse diameter (mm) | 9.8 (1.3) | 10.2 (1) | 0.001 |
| Right ventricular sphericity index | 1.68 (0.2) | 1.73 (0.2) | 0.021 |
| Left longitudinal diameter (mm) | 19.6 (3) | 19.9 (3) | 0.001 |
| Left basal transverse diameter (mm) | 10.6 (2) | 10.5 (2) | 0.001 |
| Left ventricular sphericity index | 1.86 (0.2) | 1.90 (0.2) | 0.045 |
| Right free diastolic wall thickness (mm) | 2.9 (0.3) | 2.6 (0.3) | <0.001 |
| Left free diastolic wall thickness (mm) | 2.9 (0.3) | 2.6 (0.3) | <0.001 |
| Septal diastolic wall thickness (mm) | 3.0 (0.4) | 2.8 (0.3) | 0.001 |
| Right relative wall thickness (mm) | 0.66 (0.12) | 0.56 (0.11) | <0.001 |
| Left relative wall thickness (mm) | 0.69 (0.14) | 0.58 (0.12) | <0.001 |
| Systolic function | |||
| Aortic VTI (cm2) | 9.7 (1.7) | 8.1 (1.0) | 0.001 |
| Main pulmonary VTI (cm2) | 9.2 (1.5) | 8.0 (1.0) | 0.001 |
| Tricuspid annular plane systolic excursion (mm) | 6.9 (0.9) | 5.9 (0.7) | <0.001 |
| Mitral annular plane systolic excursion (mm) | 4.9 (0.8) | 4.4 (1.1) | <0.001 |
| Isovolumetric contraction time (ms) | 32 (4) | 29 (3) | 0.014 |
| Ejection time (ms) | 172 (11) | 170 (12) | 0.242 |
| Myocardial performance index | 0.44 (0.05) | 0.40 (0.04) | 0.031 |
| Right stroke volume (mL) | 1.82 (0.6) | 1.58 (0.6) | 0.001 |
| Right cardiac output (mL/min) | 257 (87) | 221 (87) | <0.001 |
| Left stroke volume (mL) | 1.66 (0.6) | 1.35 (0.5) | <0.001 |
| Left cardiac output (mL/min) | 234 (87) | 189 (69) | <0.001 |
| Combined cardiac output (mL/min) | 492 (160) | 411 (145) | <0.001 |
| Right ejection fraction (%) | 77.8 (9.3) | 67.2 (11.3) | 0.002 |
| Right shortening fraction | 41.9 (9.1) | 34.4 (8.9) | 0.003 |
| Left ejection fraction (%) | 80.5 (8.6) | 73.7 (11.2) | 0.023 |
| Left shortening fraction | 42.9 (9.1) | 37.2 (9.9) | 0.015 |
| Diastolic function | |||
| Tricuspid E (cm/seg) | 37 (8) | 34 (7) | 0.061 |
| Tricuspid A (cm/seg) | 52 (10) | 50 (9) | 0.055 |
| Tricuspid E/A ratio | 0.71 (0.1) | 0.70 (0.1) | 0.064 |
| Mitral E (cm/seg) | 34 (8) | 32 (6) | 0.067 |
| Mitral A (cm/seg) | 48 (9) | 47 (8) | 0.108 |
| Mitral E/A ratio | 0.71 (0.1) | 0.68 (0.1) | 0.190 |
| Isovolumetric relaxation time (ms) | 43 (5) | 39 (6) | 0.031 |
| Tricuspid regurgitation (%) | 13 | 6 | <0.001 |
| Characteristic | MCDA Fetuses (n = 100) | Singleton (n = 200) | p |
|---|---|---|---|
| Doppler | |||
| Umbilical artery PI | 0.68 (0.6) | 0.15 (0.7) | 0.042 |
| Middle cerebral artery PI | −0.01 (0.6) | −0.02 (0.6) | 0.944 |
| Ductus venosus PI | 0.13 (1.1) | −0.07 (0.9) | 0.021 |
| Cardiac morphometry | |||
| Cardiac area | 0.03 (0.1) | −0.01 (0.1) | 0.579 |
| Cardiothoracic ratio | 0.07 (0.7) | 0.01 (0.8) | 0.923 |
| Left atrial area | 0.46 (0.7) | −0.03 (0.8) | 0.001 |
| Left atria-to-heart ratio | 0.57 (0.6) | 0.11 (0.8) | 0.001 |
| Left ventricular area | −0.17 (0.8) | 0.09 (0.8) | 0.019 |
| Left ventricle-to-heart ratio | −0.02 (0.9) | 0.09 (0.9) | 0.023 |
| Right atrial area | 0.26 (0.8) | −0.04 (1.0) | 0.008 |
| Right atria-to-heart ratio | 0.45 (0.7) | 0.02 (0.9) | 0.001 |
| Right ventricular area | −0.73 (0.8) | −0.05 (0.6) | 0.001 |
| Right ventricle-to-heart ratio | −0.18 (0.9) | 0.16 (0.5) | 0.001 |
| Right longitudinal diameter | −0.06 (0.6) | 0.10 (0.6) | 0.046 |
| Right basal transverse diameter | −0.01 (0.8) | 0.17 (0.9) | 0.001 |
| Right ventricular sphericity index | −0.08 (0.3) | 0.05 (0.5) | 0.036 |
| Left longitudinal diameter | −0.17 (0.9) | 0.07 (0.9) | 0.047 |
| Left basal transverse diameter | −0.23 (0.8) | −0.10 (0.8) | 0.020 |
| Left ventricular sphericity index | −0.14 (0.7) | 0.05 (0.7) | 0.045 |
| Right ventricular diastolic wall thickness | 0.78 (0.4) | 0.01 (0.6) | 0.001 |
| Left ventricular diastolic wall thickness | 0.69 (0.3) | −0.04 (0.4) | 0.001 |
| Septal diastolic thickness | 0.30 (0.6) | −0.15 (0.5) | 0.001 |
| Right relative wall thickness | 0.85 (0.9) | 0.14 (0.9) | 0.001 |
| Left relative wall thickness | 0.94 (0.9) | 0.11 (0.9) | 0.001 |
| Systolic function | |||
| Tricuspid annular plane systolic excursion | 1.36 (1.0) | 0.07 (0.6) | 0.001 |
| Mitral annular plane systolic excursion | 1.02 (1.3) | 0.00 (1.0) | 0.001 |
| Isovolumetric contraction time | 0.44 (0.4) | 0.06 (0.4) | 0.009 |
| Ejection time | −0.03 (0.4) | −0.05 (0.7) | 0.762 |
| Myocardial performance index | 0.73 (0.7) | 0.09 (0.5) | 0.008 |
| Right cardiac output | 0.55 (0.8) | −0.03 (0.9) | 0.009 |
| Left cardiac output | 0.70 (1.0) | −0,03 (1.0) | 0.045 |
| Combined cardiac output | 0.79 (1.0) | 0.08 (1.0) | 0.001 |
| Diastolic function | |||
| Isovolumetric relaxation time | 0.50 (0.6) | −0.05 (0.5) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, X.; Bennasar, M.; García-Otero, L.; Martínez-Portilla, R.J.; Valenzuela-Alcaraz, B.; Crispi, F.; Goncé, A.; Gratacós, E.; Figueras, F.; Martínez, J.M. Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta. J. Clin. Med. 2020, 9, 3602. https://doi.org/10.3390/jcm9113602
Torres X, Bennasar M, García-Otero L, Martínez-Portilla RJ, Valenzuela-Alcaraz B, Crispi F, Goncé A, Gratacós E, Figueras F, Martínez JM. Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta. Journal of Clinical Medicine. 2020; 9(11):3602. https://doi.org/10.3390/jcm9113602
Chicago/Turabian StyleTorres, Ximena, Mar Bennasar, Laura García-Otero, Raigam J. Martínez-Portilla, Brenda Valenzuela-Alcaraz, Fátima Crispi, Anna Goncé, Eduard Gratacós, Francesc Figueras, and Josep M. Martínez. 2020. "Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta" Journal of Clinical Medicine 9, no. 11: 3602. https://doi.org/10.3390/jcm9113602
APA StyleTorres, X., Bennasar, M., García-Otero, L., Martínez-Portilla, R. J., Valenzuela-Alcaraz, B., Crispi, F., Goncé, A., Gratacós, E., Figueras, F., & Martínez, J. M. (2020). Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta. Journal of Clinical Medicine, 9(11), 3602. https://doi.org/10.3390/jcm9113602







