Aspiration Thrombectomy in Patients with Acute Myocardial Infarction—5-Year Analysis Based on a Large National Registry (ORPKI)
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Frequency of TA
3.2. Periprocedural Complications according to Thrombectomy Status
3.3. Type of PCI according to Thrombectomy Status
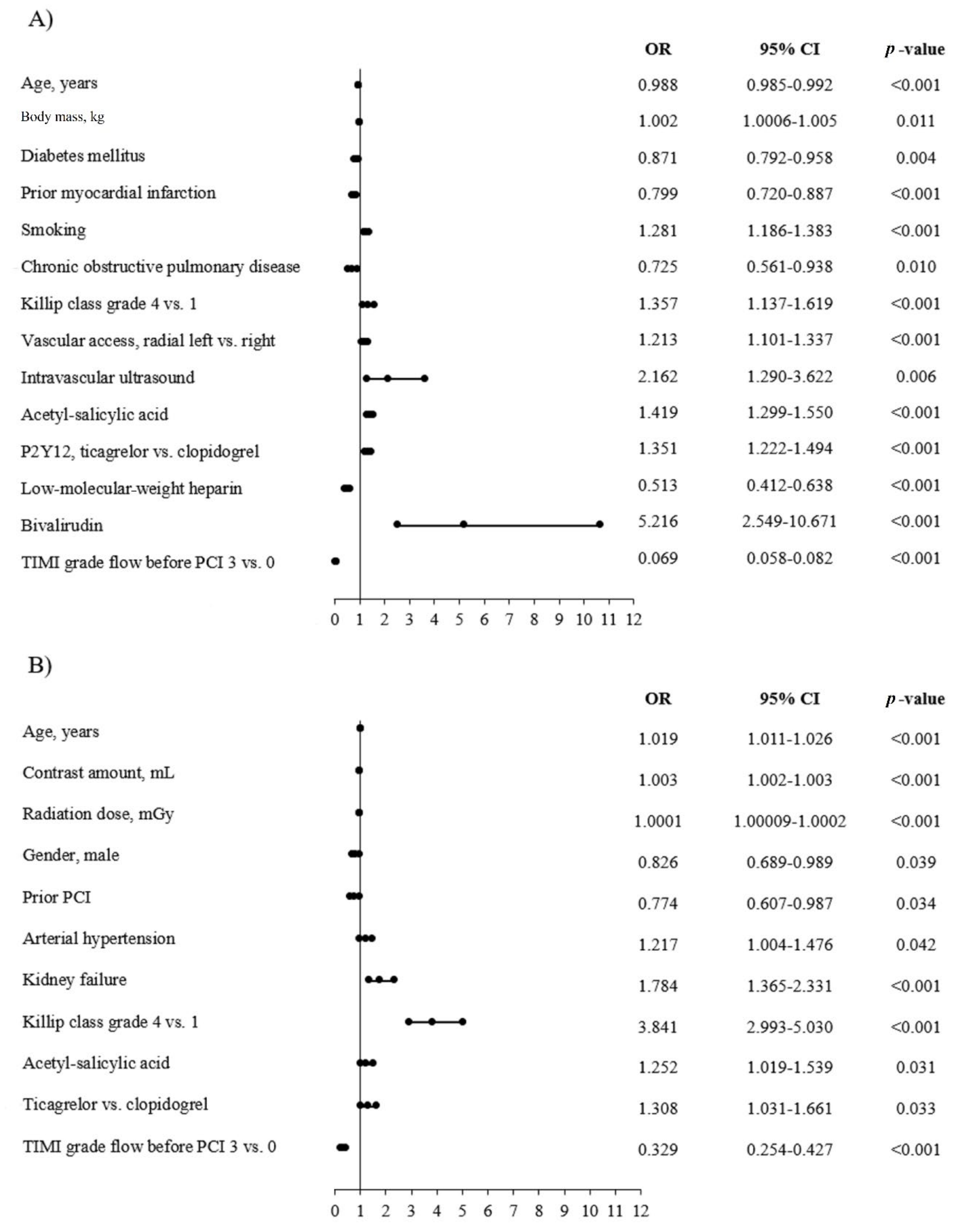
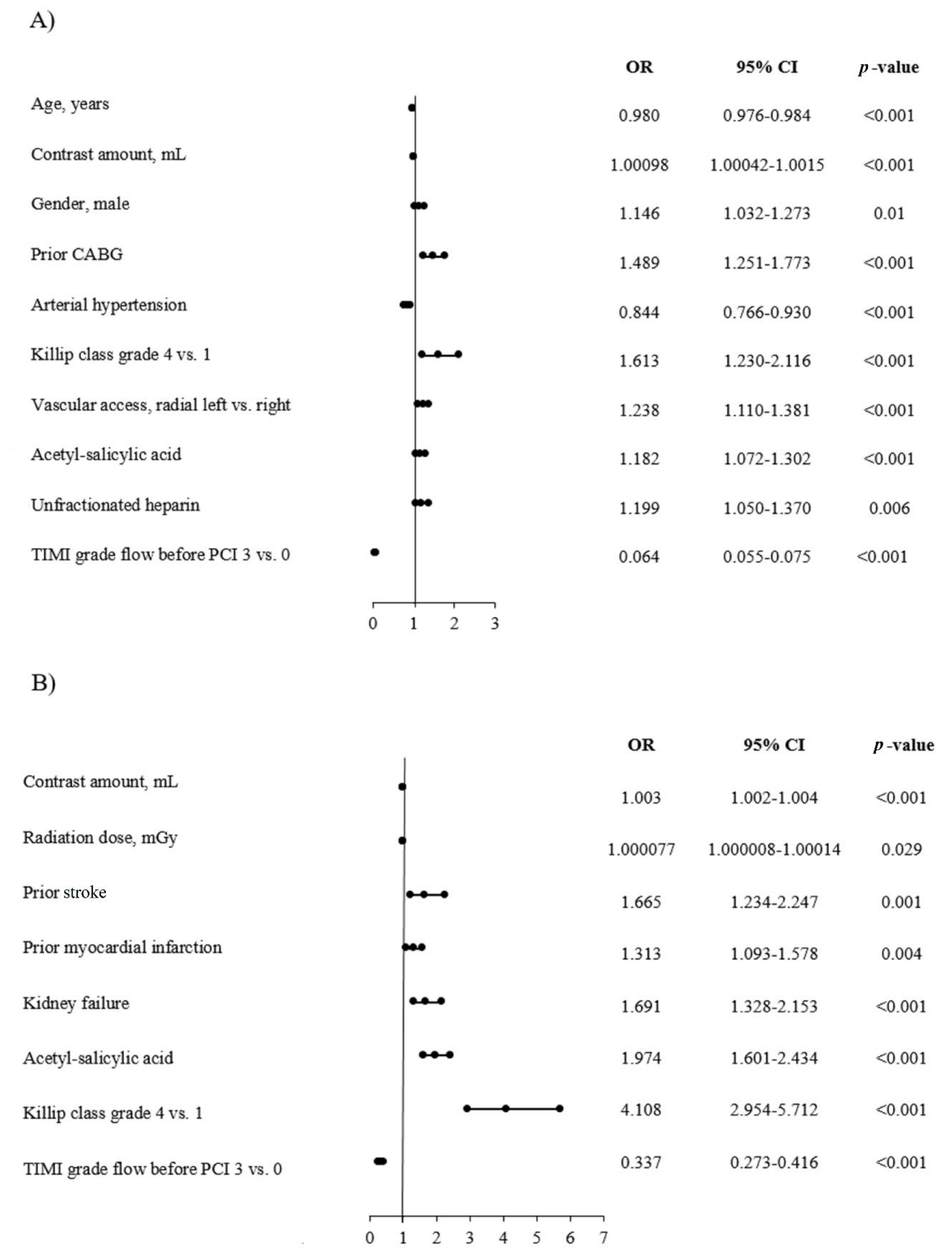
3.4. Predictors of No-Reflow and AT in the AMI Group
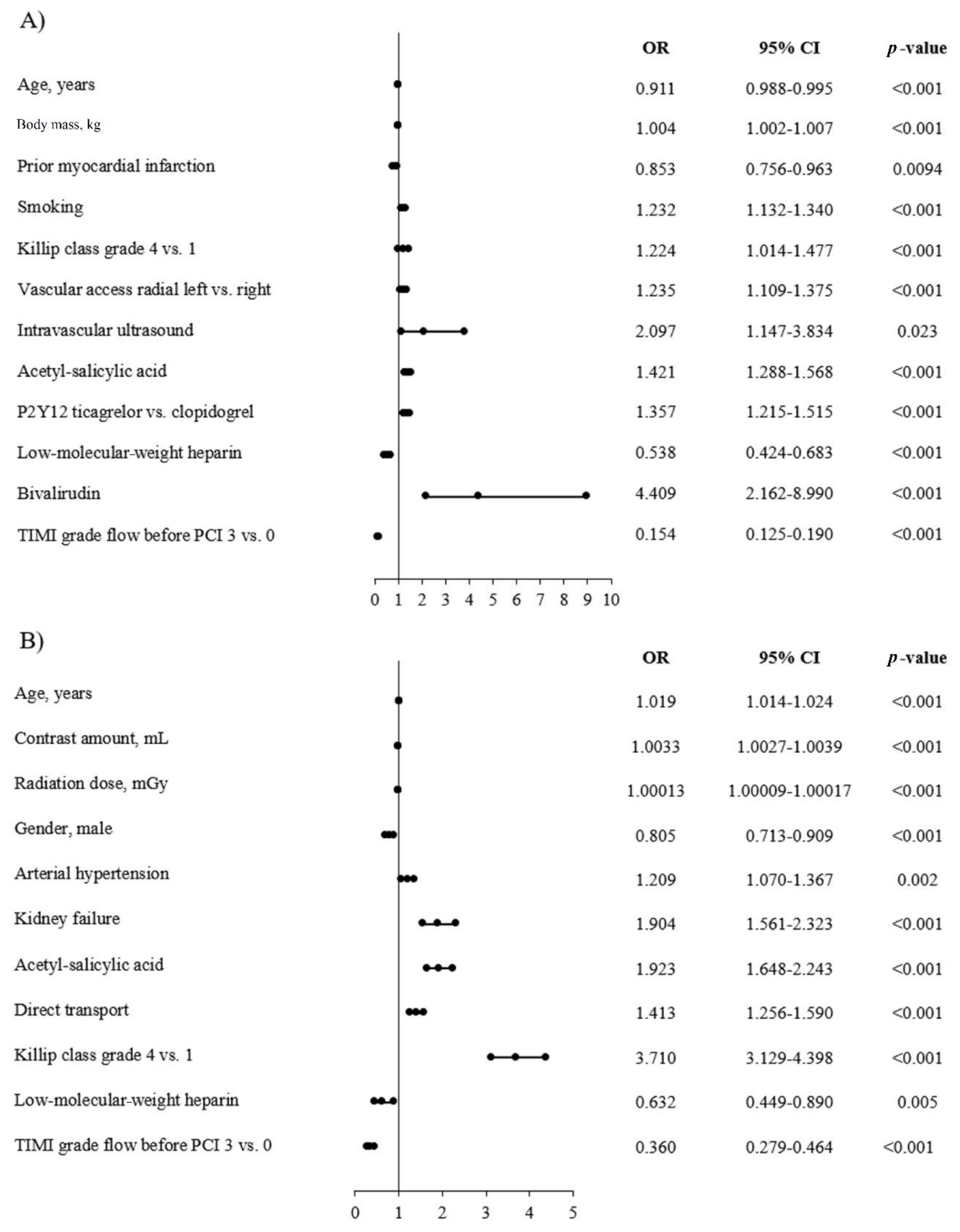
3.5. Predictors of No-Reflow and AT in the STEMI Group
3.6. Predictors of No-Reflow and AT in the NSTEMI Group
4. Discussion
4.1. Predictors of No-Reflow Phenomenon
4.2. Anitplatelet Therapy
4.3. Timing of Thrombus Aspiration
4.4. Microvascular Circulation
4.5. Thrombus Type
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, G.W.; Peterson, M.A.; Lansky, A.J.; Dangas, G.; Mehran, R.; Leon, M.B. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 591–597. [Google Scholar] [CrossRef]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular Obstruction and the No-Reflow Phenomenon After Percutaneous Coronary Intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, P.J.; Svilaas, T.; Van Der Horst, I.C.; Diercks, G.F.H.; Fokkema, M.L.; De Smet, B.J.G.L.; Heuvel, A.F.M.V.D.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.-S.; et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): A 1-year follow-up study. Lancet 2008, 371, 1915–1920. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Rokoss, M.J.; Gao, P.; Meeks, B.; Kedev, S.; Stankovic, G.; Moreno, R.; Gershlick, A.H.; et al. TOTAL Investigators. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016, 387, 127–135. [Google Scholar] [CrossRef]
- Olivecrona, G.K.; Lagerquist, B.; Fröbert, O.; Gudnason, T.; Maeng, M.; Råmunddal, T.; Haupt, J.; Kellerth, T.; Stewart, J.; Sarno, G.; et al. Impact of thrombus aspiration during ST-Elevation Myocardial Infarction: A six month composite endpoint and risk of stroke analyses of the TASTE trial. BMC Cardiovasc. Disord. 2016, 16, 62. [Google Scholar] [CrossRef]
- Zabojszcz, M.; Januszek, R.; Siudak, Z.; Janion-Sadowska, A.; Jędrychowska, M.; Pawlik, A.; Tokarek, T.; Staszczak, B.; Malinowski, K.P.; Bartuś, S.; et al. Association between the mortality rate and operator volume in patients undergoing emergency or elective percutaneous coronary interventions. Kardiol Pol. 2020, 78, 138–146. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2017, 39, 119–177. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 575. [Google Scholar] [CrossRef]
- Januszek, R.; Siudak, Z.; Reczuch, K.; Dobrzycki, S.; Lesiak, M.; Legutko, J.; Kleczyński, P.; Rzeszutko, Ł.; Dudek, D.; Bartuś, S.; et al. Current trends and procedural outcomes in the era of rotational atherectomy expansion in Poland in the period 2014-2017 (based on the nationwide ORPKI registry). Adv. Interv. Cardiol. 2019, 15, 158–166. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Scalise, R.F.M.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 39, 213–260. [Google Scholar] [CrossRef]
- Bahrmann, P.; Rach, J.; Desch, S.; Schuler, G.C.; Thiele, H. Incidence and distribution of occluded culprit arteries and impact of coronary collaterals on outcome in patients with non-ST-segment elevation myocardial infarction and early invasive treatment strategy. Clin. Res. Cardiol. 2010, 100, 457–467. [Google Scholar] [CrossRef]
- McEntegart, M.B.; Kirtane, A.J.; Cristea, E.; Brener, S.; Mehran, R.; Fahy, M.; Moses, J.W.; Stone, G.W. Intraprocedural Thrombotic Events During Percutaneous Coronary Intervention in Patients with Non–ST-Segment Elevation Acute Coronary Syndromes Are Associated with Adverse Outcomes: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J. Am. Coll. Cardiol. 2012, 59, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Kastrati, A. Mechanical strategies to enhance myocardial salvage during primary percutaneous coronary intervention in patients with STEMI. EuroIntervention 2016, 12, 319–328. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2015, 37, 1024–1033. [Google Scholar] [CrossRef]
- Lønborg, J.; Kelbæk, H.; Helqvist, S.; Holmvang, L.; Jørgensen, E.; Saunamäki, K.; Kløvgaard, L.; Kaltoft, A.; Bøtker, H.E.; Lassen, J.F.; et al. The impact of distal embolization and distal protection on long-term outcome in patients with ST elevation myocardial infarction randomized to primary percutaneous coronary intervention–results from a randomized study. Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 180–188. [Google Scholar] [CrossRef]
- Barbato, E.; Marco, J.; Wijns, W. Direct stenting. Eur. Heart J. 2003, 24, 394–403. [Google Scholar] [CrossRef][Green Version]
- Romaguera, R.; Gracida, M.; Teruel, L.; Gómez-Hospital, J.A.; Sánchez-Elvira, G.; Gomez-Lara, J.; Ferreiro, J.L.; Roura, G.; Homs, S.; Cequier, Á. MGuard Mesh? Covered Stent for Treatment of ST?Segment Elevation Myocardial Infarction with High Thrombus Burden Despite Manual Aspiration. J. Interv. Cardiol. 2013, 26, 1–7. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef]
- Ahn, S.G.; Hung, O.Y.; Lee, J.-W.; Lee, J.H.; Youn, Y.J.; Ahn, M.-S.; Kim, J.-Y.; Yoo, B.-S.; Lee, S.-H.; Yoon, J.H.; et al. Combination of the Thermodilution-Derived Index of Microcirculatory Resistance and Coronary Flow Reserve Is Highly Predictive of Microvascular Obstruction on Cardiac Magnetic Resonance Imaging After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2016, 9, 793–801. [Google Scholar] [CrossRef]
- Sardu, C.; Barbieri, M.; Balestrieri, M.L.; Siniscalchi, M.; Paolisso, P.; Calabrò, P.; Minicucci, F.; Signoriello, G.; Portoghese, M.; Mone, P.; et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: Clinical outcomes at 1-year follow-up. Cardiovasc Diabetol. 2018, 17, 152. [Google Scholar] [CrossRef]
- Sirker, A.; Mamas, M.; Kwok, C.S.; Kontopantelis, E.; Ludman, P.; Hildick-Smith, D. British Cardiovascular Intervention Society (BCIS). Outcomes from Selective Use of Thrombectomy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction: An Analysis of the British Cardiovascular Intervention Society/National Institute for Cardiovascular Outcomes Research (BCIS-NICOR) Registry, 2006–2013. JACC Cardiovasc. Interv. 2016, 9, 126–134. [Google Scholar] [CrossRef]
- Lin, M.-S.; Wu, L.-S.; Cheng, N.-J.; Lin, P.-C.; Chang, C.-J. Thrombus aspiration complicated by systemic embolization in patients with acute myocardial infarction. Circ. J. 2008, 73, 1356–1358. [Google Scholar] [CrossRef]
- Costopoulos, C.; A Gorog, D.; Di Mario, C.; Kukreja, N. Use of thrombectomy devices in primary percutaneous coronary intervention: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 163, 229–241. [Google Scholar] [CrossRef]
- Schmitt, J.; Duray, G.; Gersh, B.J.; Hohnloser, S.H. Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. Eur. Heart J. 2008, 30, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Huo, T.; Bhatt, D.L.; Bavry, A.A. TCT-227 Is aspiration thrombectomy beneficial in patients undergoing primary percutaneous coronary intervention? An updated meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2015, 66, B88–B89. [Google Scholar] [CrossRef][Green Version]
- Kilic, S.; Fabris, E.; Hof, A.W.V.; Hamm, C.W.; Lapostolle, F.; Lassen, J.F.; Tsatsaris, A.; Diallo, A.; Vicaut, E.; Montalescot, G. ATLANTIC Investigators. Thrombus aspiration and prehospital ticagrelor administration in ST-elevation myocardial infarction: Findings from the ATLANTIC trial. Am. Heart J. 2018, 196, 1–8. [Google Scholar] [CrossRef]
- Ghatak, A.; Singh, V.; Shantha, G.P.S.; Badheka, A.; Patel, N.; Alfonso, C.E.; Biswas, M.; Pancholy, S.B.; Grines, C.; O’Neill, W.W.; et al. Aspiration Thrombectomy in Patients Undergoing Primary Angioplasty for ST Elevation Myocardial Infarction: An Updated Meta-Analysis. J. Interv. Cardiol. 2015, 28, 503–513. [Google Scholar] [CrossRef]
- Jolly, S.S.; James, S.; Džavík, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Response by Jolly et al to Letters Regarding Article, "Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration". Circulation 2017, 135, e1103–e1104. [Google Scholar] [CrossRef]
- Wong, D.T.; Puri, R.; Richardson, J.D.; Worthley, M.I.; Worthley, S.G.; Puri, R.; Worthley, M.I. Myocardial ‘no-reflow’—Diagnosis, pathophysiology and treatment. Int. J. Cardiol. 2013, 167, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Tomaszuk-Kazberuk, A.; Sobkowicz, B.; Kaminski, K.A.; Gugala, K.; Mezynski, G.; Dobrzycki, S.; Lewczuk, A.; Kazberuk, W.; Musial, W.J. Myocardial perfusion assessed by contrast echocardiography correlates with angiographic perfusion parameters in patients with a first acute myocardial infarction successfully treated with angioplasty. Can. J. Cardiol. 2008, 24, 633–639. [Google Scholar] [CrossRef][Green Version]
- Ramjane, K.; Han, L.; Jin, C. The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp. Clin. Cardiol. 2008, 13, 121–128. [Google Scholar] [PubMed]
- Soeda, T.; Shibutani, S.; Ong, D.S.; Vergallo, R.; Minami, Y.; Higuma, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Lee, H.; et al. Morphological predictors for no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur. Hear. J.-Cardiovasc. Imaging 2016, 18, 103–110. [Google Scholar] [CrossRef]
- Fajar, J.K.; Heriansyah, T.; Rohman, M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J. 2018, 70, S406–S418. [Google Scholar] [CrossRef]
- Yang, L.; Cong, H.; Lu, Y.; Chen, X.; Liu, Y. Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine 2020, 99, e20152. [Google Scholar] [CrossRef]
- Sharma, V.; Jolly, S.S.; Hamid, T.; Sharma, D.; Chiha, J.; Chan, W.; Fuchs, F.; Bui, S.; Gao, P.; Kassam, S.; et al. Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: Insights from the TOTAL trial. Eur. Heart J. 2016, 37, 1891–1898. [Google Scholar] [CrossRef]
- Hachinohe, D.; Jeong, J.-O.; Saito, S.; Kim, M.C.; Cho, K.H.; Ahmed, K.; Hwang, S.H.; Lee, M.-G.; Sim, D.S.; Park, K.-H.; et al. Korea Acute Myocardial Infarction Registry Investigators. Clinical impact of thrombus aspiration during primary percutaneous coronary intervention: Results from Korea Acute Myocardial Infarction Registry. J. Cardiol. 2012, 59, 249–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.-H.E.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Bin Gwag, H.; Kim, E.K.; Park, T.K.; Lee, J.M.; Yang, J.H.; Bin Song, Y.; Choi, J.; Choi, S.; Lee, S.H.; Chang, S.-A.; et al. Cardioprotective Effects of Intracoronary Morphine in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention: A Prospective, Randomized Trial. J. Am. Heart Assoc. 2017, 6, 005426. [Google Scholar] [CrossRef]
- Sim, D.S.; Jeong, J.-O.; Ahn, Y.; Kim, Y.-J.; Chae, S.C.; Hong, T.J.; Seo, H.S.; Chae, J.K.; Kim, C.-J.; Cho, M.-C.; et al. Other Korea Acute Myocardial Infarction Registry (KAMIR) Investigators. Manual thrombus aspiration during primary percutaneous coronary intervention: Impact of total ischemic time. J. Cardiol. 2017, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ikari, Y.; Sakurada, M.; Kozuma, K.; Kawano, S.; Katsuki, T.; Kimura, K.; Suzuki, T.; Yamashita, T.; Takizawa, A.; Misumi, K.; et al. VAMPIRE Investigators. Upfront Thrombus Aspiration in Primary Coronary Intervention for Patients With ST-Segment Elevation Acute Myocardial Infarction: Report of the VAMPIRE (VAcuuM asPIration thrombus REmoval) trial. JACC Cardiovasc. Interv. 2008, 1, 424–431. [Google Scholar] [CrossRef]
- De Vita, M.; Burzotta, F.; Porto, I.; Dudek, D.; Lefèvre, T.; Trani, C.; Mielecki, W.; Niccoli, G.; Biondi-Zoccai, G.G.L.; Crea, F. Thrombus aspiration in ST elevation myocardial infarction: Comparative efficacy in patients treated early and late after onset of symptoms. Heart 2010, 96, 1287–1290. [Google Scholar] [CrossRef][Green Version]
- De Rosa, S.; Caiazzo, G.; Torella, D.; Indolfi, C. Aspiration Thrombectomy: An easily forgiven latecomer. J. Am. Coll. Cardiol. 2014, 63, 2052–2053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumbhani, D.J.; Bavry, A.A.; Desai, M.Y.; Bangalore, S.; Bhatt, D.L. Role of Aspiration and Mechanical Thrombectomy in Patients with Acute Myocardial Infarction Undergoing Primary Angioplasty: An updated meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2013, 62, 1409–1418. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, M.-J.; Ko, K.-Y.; Park, J.-H.; Baek, Y.-S.; Sung-Woo, K.; Shin, S.-H.; Woo, S.-I.; Kim, D.-H.; Suh, Y.J.; et al. Mechanical and Pharmacological Revascularization Strategies for Prevention of Microvascular Dysfunction in ST-Segment Elevation Myocardial Infarction: Analysis from Index of Microcirculatory Resistance Registry Data. J. Interv. Cardiol. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
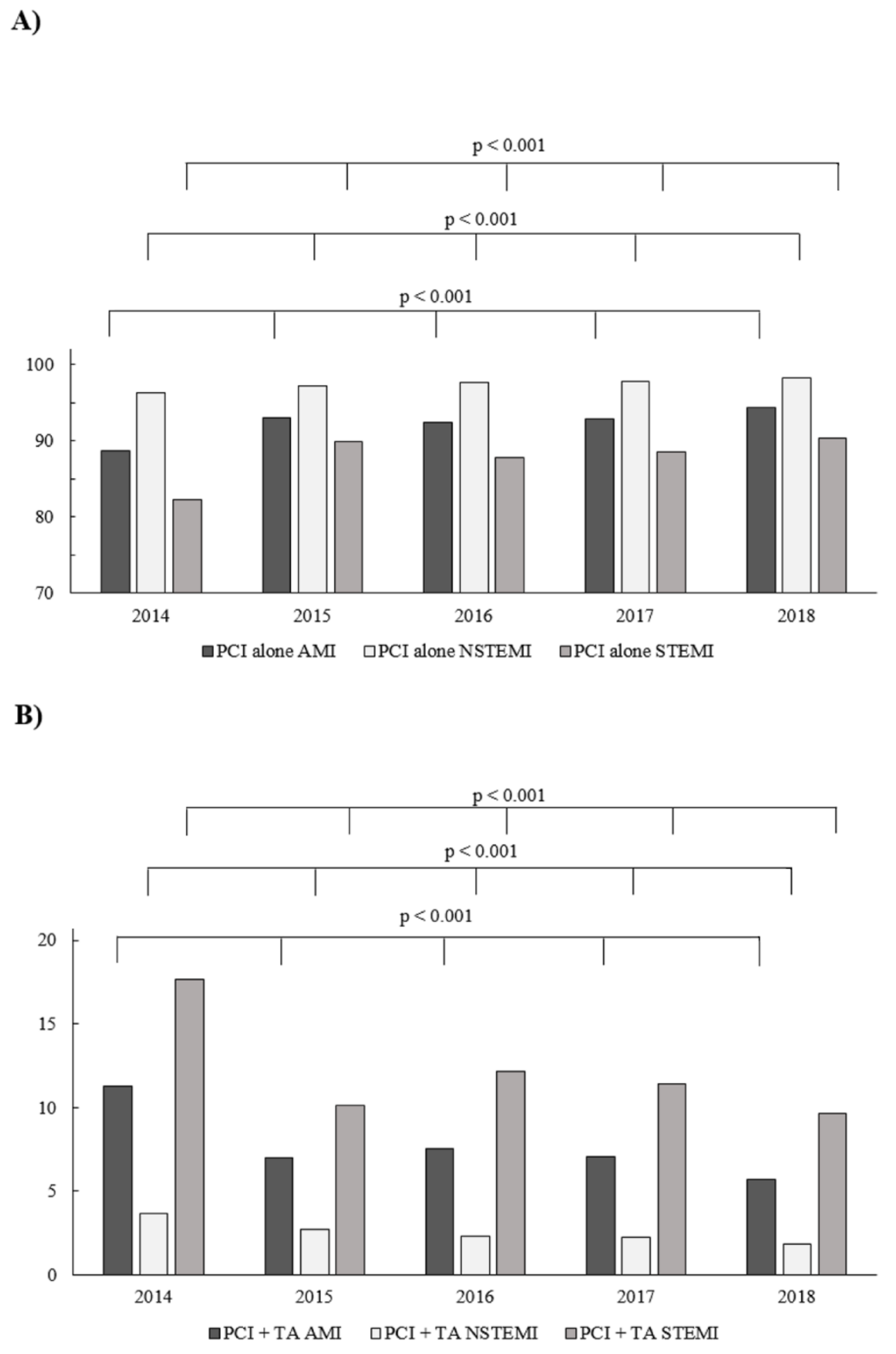
| Years | p-Value | |||
|---|---|---|---|---|
| Level 1 | Level 2 | AMI | NSTEMI | STEMI |
| 2015 | 2014 | <0.001 | <0.001 | <0.001 |
| 2016 | 2014 | <0.001 | <0.001 | <0.001 |
| 2016 | 2015 | 0.001 | 0.003 | <0.001 |
| 2017 | 2014 | <0.001 | <0.001 | <0.001 |
| 2017 | 2015 | 0.46 | 0.001 | <0.001 |
| 2017 | 2016 | 0.019 | 0.83 | 0.015 |
| 2018 | 2014 | <0.001 | <0.001 | <0.001 |
| 2018 | 2015 | <0.001 | <0.001 | 0.072 |
| 2018 | 2016 | <0.001 | 0.001 | <0.001 |
| 2018 | 2017 | <0.001 | 0.002 | <0.001 |
| Type of AMI | Direct Transport | NRP | p-Value | ||
|---|---|---|---|---|---|
| Total | Present | Absent | |||
| NSTEMI | Total | 90,849 | 607 | 91,456 | 0.038 |
| Absent | 85,191 (93.15) | 552 (90.94) | 84,643 (93.17) | 0.03 | |
| Present | 6261 (6.85) | 55 (9.06) | 6206 (6.83) | 0.035 | |
| STEMI | Total | 105,362 | 1420 | 103,942 | <0.001 |
| Absent | 80,007 (75.94) | 928 (65.35) | 79,079 (76.08) | <0.001 | |
| Present | 24,863 (23.92) | 492 (34.65) | 25,355 (24.06) | <0.001 | |
| Variable (%) | Non-Thrombectomy Group | Thrombectomy Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | NSTEMI | STEMI | p-Value | Overall | NSTEMI | STEMI | p-Value | |
| N = 200,991 | N = 98,492 | N = 102,499 | N = 16,777 | N = 2570 | N = 14,207 | |||
| No-reflow | 0.82 | 0.58 | 1.06 | <0.001 | 2.75 * | 3.07 * | 2.69 * | 0.27 |
| Puncture-site bleeding | 0.13 | 0.14 | 0.12 | 0.11 | 0.14 | 0.19 | 0.13 | 0.41 |
| Cardiac arrest | 1.11 | 0.62 | 1.59 | <0.001 | 2.49 * | 1.36 * | 2.69 * | <0.001 |
| Allergic reaction | 0.1 | 0.06 | 0.13 | <0.001 | 0.45 * | 0.23 * | 0.49 * | 0.05 |
| CAP | 0.19 | 0.2 | 0.18 | 0.41 | 0.2 | 0.39 * | 0.16 | 0.03 |
| Stroke | 0.01 | 0.02 | 0.02 | 0.49 | 0.03 * | 0.16 * | 0.01 | 0.001 |
| Dissection | 0.14 | 0.14 | 0.14 | 0.9 | 0.07 * | 0.16 | 0.05 * | 0.08 |
| Stent implantation | 90.25 | 89.07 | 91.4 | <0.001 | 91.19 | 86.59 * | 91.96 | <0.001 |
| Stent type | <0.001 | 0.0016 | ||||||
| Drug-eluting stent | 87.19 | 86.41 | 87.95 | 87.89 | 82.88 * | 88.73 | ||
| Bare-metal stent | 2.32 | 1.91 | 2.73 | 2.21 | 1.83 | 2.28 | ||
| Bioresorbable scaffold | 0.69 | 0.69 | 0.69 | 1.04 | 1.83 * | 0.91 | ||
| No stent used | 9.8 | 10.98 | 8.63 | 8.85 * | 13.47 | 8.08 | ||
| Variable | No-Reflow | Aspiration Thrombectomy | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age, years | 1.018 | 1.014–1.022 | <0.001 | 0.976 | 0.975–0.977 | <0.001 |
| Weight, kg | 0.999 | 0.996–1.001 | 0.53 | 1.004 | 1.004–1.006 | <0.001 |
| Contrast amount, mL | 1.004 | 1.003–1.004 | <0.001 | 1.0002 | 1.0001–1.0004 | 0.06 |
| Radiation exposure, mGy | 1.0002 | 1.0002–1.0003 | <0.001 | 1.0001 | 1.0001–1.0001 | <0.0001 |
| Gender, male | 0.786 | 0.720–0.859 | <0.001 | 1.214 | 1.173–1.257 | <0.001 |
| Diabetes mellitus | 1.303 | 1.183–1.436 | <0.001 | 0.718 | 0.689–0.749 | <0.001 |
| Prior stroke | 1.974 | 1.667–2.338 | <0.001 | 0.866 | 0.793–0.946 | 0.001 |
| Prior myocardial infarction | 0.573 | 0.547–0.600 | <0.001 | |||
| Prior percutaneous coronary intervention | 0.729 | 0.649–0.820 | <0.001 | 0.555 | 0.530–0.581 | <0.001 |
| Prior CABG | 1.266 | 1.039–1.543 | 0.018 | 0.574 | 0.519–0.636 | <0.001 |
| Smoking | 1.131 | 1.027–1.244 | 0.011 | 1.565 | 1.513–1.618 | <0.001 |
| Arterial hypertension | 1.26 | 1.147–1.384 | <0.001 | 0.785 | 0.760–810 | <0.001 |
| Kidney failure | 2.25 | 1.967–2.573 | <0.001 | 0.594 | 0.546–0.646 | <0.001 |
| Chronic obstructive pulmonary disease | 1.698 | 1.347–2.141 | <0.001 | 0.754 | 0.663–0.858 | <0.001 |
| Killip-Kimball class | ||||||
| -class 4 vs. 1 | 5.683 | 4.932–6.548 | <0.001 | 1.955 | 1.803–2.119 | <0.001 |
| Cardiac arrest before admission | 2.36 | 2.009–2.773 | <0.001 | 1.877 | 1.753–2.010 | <0.001 |
| Vascular access | ||||||
| Femoral vs. radial right | 1.368 | 1.242–1.508 | <0.001 | 1.093 | 1.053–1.134 | <0.001 |
| Coronary angiography | ||||||
| MVD + LMCA vs. SVD | 1.403 | 1.162–1.693 | <0.001 | 0.52 | 0.474–0.570 | <0.001 |
| Fractional flow reserve | 0.225 | 0.135–0.376 | <0.001 | |||
| Intravascular ultrasound | 0.805 | 0.652–0.995 | 0.04 | |||
| Rotablation | 0.269 | 0.148–0.489 | <0.001 | |||
| Acetyl-salicylic acid | 1.295 | 1.182–1.420 | <0.001 | 1.123 | 1.084–1.162 | <0.001 |
| Unfractionated heparin | 1.164 | 1.037–1.308 | 0.01 | 1.063 | 1.020–1.108 | 0.003 |
| P2Y12 inhibitor | ||||||
| Ticagrelor vs. clopidogrel | 1.29 | 1.040–1.601 | 0.02 | 1.6 | 1.467–1.745 | <0.001 |
| Thrombolysis | 1.505 | 0.778–2.911 | 0.47 | 1.96 | 1.562–2.460 | <0.001 |
| Glycoprotein IIB/IIIa inhibitor 1 vs. 0 | 4.544 | 4.325–4.775 | <0.001 | |||
| TIMI flow grade before PCI 3 vs. 0 | 0.265 | 0.232–0.302 | <0.001 | 0.061 | 0.056–0.066 | <0.001 |
| Variable | No-Reflow | Aspiration Thrombectomy | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age, years | 1.028 | 1.023–1.032 | <0.001 | 0.985 | 0.983–1.015 | <0.001 |
| Weight, kg | 1.005 | 1.004–1.006 | <0.001 | |||
| Contrast amount, mL | 1.004 | 1.003–1.004 | <0.001 | |||
| Radiation exposure, mGy | 1.0002 | 1.0002–1.0003 | <0.001 | 1.0001 | 1.0001–1.0001 | <0.001 |
| Gender, male | 0.703 | 0.633–0.781 | <0.001 | 1.143 | 1.100–1.188 | <0.001 |
| Diabetes mellitus | 1.483 | 1.316–1.672 | <0.001 | 0.860 | 0.820–0.902 | <0.001 |
| Prior stroke | 1.941 | 1.559–2.416 | <0.001 | |||
| Prior myocardial infarction | 0.703 | 0.666–0.741 | <0.001 | |||
| Prior percutaneous coronary intervention | 0.725 | 0.621–0.847 | <0.001 | 0.659 | 0.624–0.695 | <0.001 |
| Prior CABG | 1.403 | 1.033–1.906 | 0.038 | 0.653 | 0.566–0.754 | <0.001 |
| Smoking | 1.421 | 1.370–1.475 | <0.001 | |||
| Arterial hypertension | 1.404 | 1.258–1.568 | <0.001 | 0.943 | 0.910–0.978 | 0.001 |
| Kidney failure | 3.133 | 2.630–3.732 | <0.001 | |||
| Chronic obstructive pulmonary disease | 2.228 | 1.672–2.968 | <0.001 | |||
| Hypothermia | 1.675 | 1.223–2.295 | 0.002 | |||
| Direct transport | 1.686 | 1.510–1.882 | <0.001 | 1.233 | 1.185–1.283 | <0.001 |
| Killip-Kimball class -class 4 vs. 1 | 4.948 | 4.217–5.807 | <0.001 | 1.485 | 1.362–1.618 | <0.001 |
| Cardiac arrest before admission | 2.114 | 1.772–2.521 | <0.001 | 1.441 | 1.340–1.550 | <0.001 |
| Vascular access Femoral vs. radial right | 1.270 | 1.132–1.425 | <0.001 | 1.018 | 0.977–1.060 | <0.001 |
| Coronary angiography MVD + LMCA vs. SVD | 1.534 | 1.214–1.938 | <0.001 | 0.580 | 0.522–0.644 | <0.001 |
| Fractional flow reserve | 0.236 | 0.126–0.444 | <0.001 | |||
| Rotablation | 0.262 | 0.123–0.558 | <0.001 | |||
| Acetyl-salicylic acid | 1.400 | 1.254–1.563 | <0.001 | 1.207 | 1.161–1.255 | <0.001 |
| Unfractionated heparin | 1.312 | 1.141–1.510 | <0.001 | 1.150 | 1.099–1.203 | <0.001 |
| P2Y12 inhibitor Ticagrelor vs. clopidogrel | 1.218 | 0.945–1.569 | <0.001 | 1.391 | 1.262–1.532 | <0.001 |
| Low molecular weight heparin | 0.861 | 0.782–0.947 | 0.001 | |||
| Glycoprotein IIB/IIIa inhibitor 1 vs. 0 | 2.010 | 1.700–2.377 | <0.001 | 3.283 | 3.109–3.467 | <0.001 |
| TIMI flow grade before PCI 3 vs. 0 | 0.254 | 0.205 | 0.316 | 0.108 | 0.098–0.118 | <0.001 |
| Variable | No-Reflow | Aspiration Thrombectomy | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age, years | 0.970 | 0.967–0.973 | <0.001 | |||
| Weight, kg | 1.006 | 1.004–1.008 | <0.001 | |||
| Contrast amount, mL | 1.004 | 1.003–1.004 | <0.001 | 1.0017 | 1.0012–1.0021 | <0.001 |
| Radiation exposure, mGy | 1.0002 | 1.0002–1.0003 | <0.001 | 1.0001 | 1.0001–1.0002 | <0.001 |
| Gender, male | 1.435 | 1.314–1.569 | <0.001 | |||
| Diabetes mellitus | 1.283 | 1.085–1.516 | 0.003 | 0.765 | 0.695–0.841 | <0.001 |
| Prior stroke | 2.314 | 1.771–3.023 | <0.001 | 0.804 | 0.649–0.996 | 0.045 |
| Prior myocardial infarction | 1.515 | 1.288–1.782 | <0.001 | 0.708 | 0.642–0.780 | <0.001 |
| Prior percutaneous coronary intervention | 0.652 | 0.589–0.722 | <0.001 | |||
| Prior CABG | 1.628 | 1.251–2.118 | <0.001 | 1.276 | 1.101–1.480 | 0.001 |
| Smoking | 1.211 | 1.015–1.444 | 0.032 | 1.540 | 1.415–1.677 | <0.001 |
| Arterial hypertension | 1.267 | 1.058–1.518 | 0.009 | 0.805 | 0.741–0.875 | <0.001 |
| Kidney failure | 2.128 | 1.719–2.635 | <0.001 | 0.600 | 0.501–717 | <0.001 |
| COPD | ||||||
| Hypothermia | 3.398 | 1.356–8.514 | 0.026 | |||
| Direct transport | 1.358 | 1.028–1.795 | 0.03 | 1.178 | 1.017–1.365 | 0.031 |
| Killip-Kimball class - class 4 vs. 1 | 5.648 | 4.119–7.745 | <0.001 | 1.854 | 1.436–2.394 | <0.001 |
| Cardiac arrest before admission | 1.956 | 1.298–2.948 | 0.001 | 1.676 | 1.337–2.100 | <0.001 |
| Vascular access Femoral vs. radial right | 1.459 | 1.220–1.744 | <0.001 | 1.033 | 0.939–1.136 | <0.001 |
| Coronary angiography MVD + LMCA vs. SVD | 1.484 | 1.079–2.042 | 0.015 | 0.684 | 0.559–0.837 | <0.001 |
| Fractional flow reserve | 0.390 | 0.161–0.943 | 0.013 | |||
| Acetyl-salicylic acid | 1.180 | 1.0003–1.392 | 0.049 | 1.099 | 1.010–1.197 | 0.029 |
| Unfractionated heparin | 1.204 | 1.076–1.347 | <0.001 | |||
| P2Y12 inhibitor Ticagrelor vs. clopidogrel | 1.491 | 1.193–1.865 | 0.005 | |||
| Thrombolysis | 7.005 | 4.106–11.950 | <0.001 | |||
| Glycoprotein IIB/IIIa inhibitor 1 vs. 0 | 3.768 | 2.820–5.033 | <0.001 | 6.253 | 5.500–7.110 | <0.001 |
| TIMI flow grade before PCI 3 vs. 0 | 0.330 | 0.276–0.399 | <0.001 | 0.058 | 0.050–0.067 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszek, R.; Siudak, Z.; Malinowski, K.P.; Wojdyła, R.; Mika, P.; Wańha, W.; Kameczura, T.; Surdacki, A.; Wojakowski, W.; Legutko, J.; et al. Aspiration Thrombectomy in Patients with Acute Myocardial Infarction—5-Year Analysis Based on a Large National Registry (ORPKI). J. Clin. Med. 2020, 9, 3610. https://doi.org/10.3390/jcm9113610
Januszek R, Siudak Z, Malinowski KP, Wojdyła R, Mika P, Wańha W, Kameczura T, Surdacki A, Wojakowski W, Legutko J, et al. Aspiration Thrombectomy in Patients with Acute Myocardial Infarction—5-Year Analysis Based on a Large National Registry (ORPKI). Journal of Clinical Medicine. 2020; 9(11):3610. https://doi.org/10.3390/jcm9113610
Chicago/Turabian StyleJanuszek, Rafał, Zbigniew Siudak, Krzysztof P. Malinowski, Roman Wojdyła, Piotr Mika, Wojciech Wańha, Tomasz Kameczura, Andrzej Surdacki, Wojciech Wojakowski, Jacek Legutko, and et al. 2020. "Aspiration Thrombectomy in Patients with Acute Myocardial Infarction—5-Year Analysis Based on a Large National Registry (ORPKI)" Journal of Clinical Medicine 9, no. 11: 3610. https://doi.org/10.3390/jcm9113610
APA StyleJanuszek, R., Siudak, Z., Malinowski, K. P., Wojdyła, R., Mika, P., Wańha, W., Kameczura, T., Surdacki, A., Wojakowski, W., Legutko, J., & Bartuś, S. (2020). Aspiration Thrombectomy in Patients with Acute Myocardial Infarction—5-Year Analysis Based on a Large National Registry (ORPKI). Journal of Clinical Medicine, 9(11), 3610. https://doi.org/10.3390/jcm9113610







