Lyso-Gb3 Increases αvβ3 Integrin Gene Expression in Cultured Human Podocytes in Fabry Nephropathy
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture and Reagents
2.2. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.3. Western Blot
2.4. Statistical Analysis
3. Results
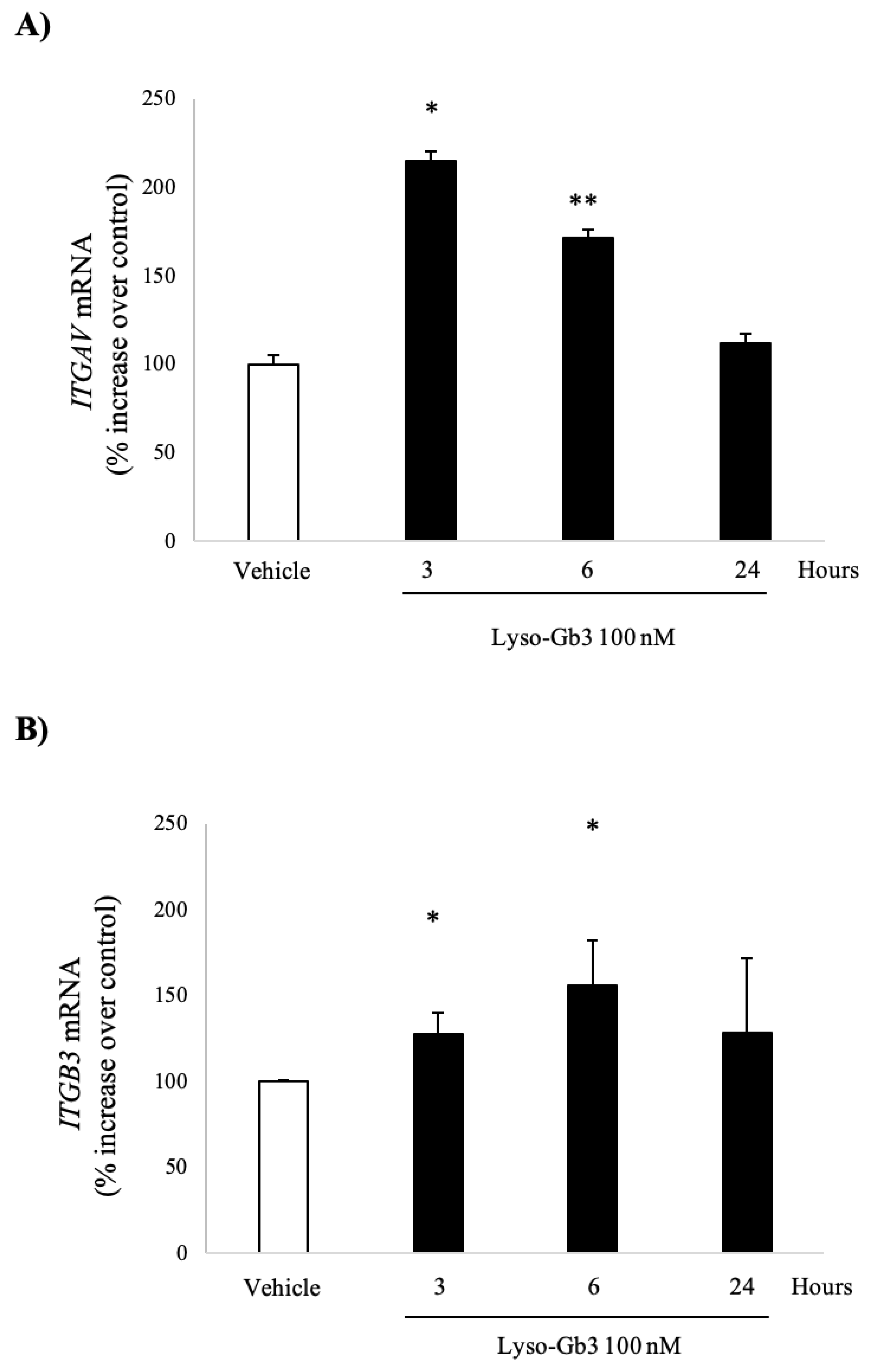
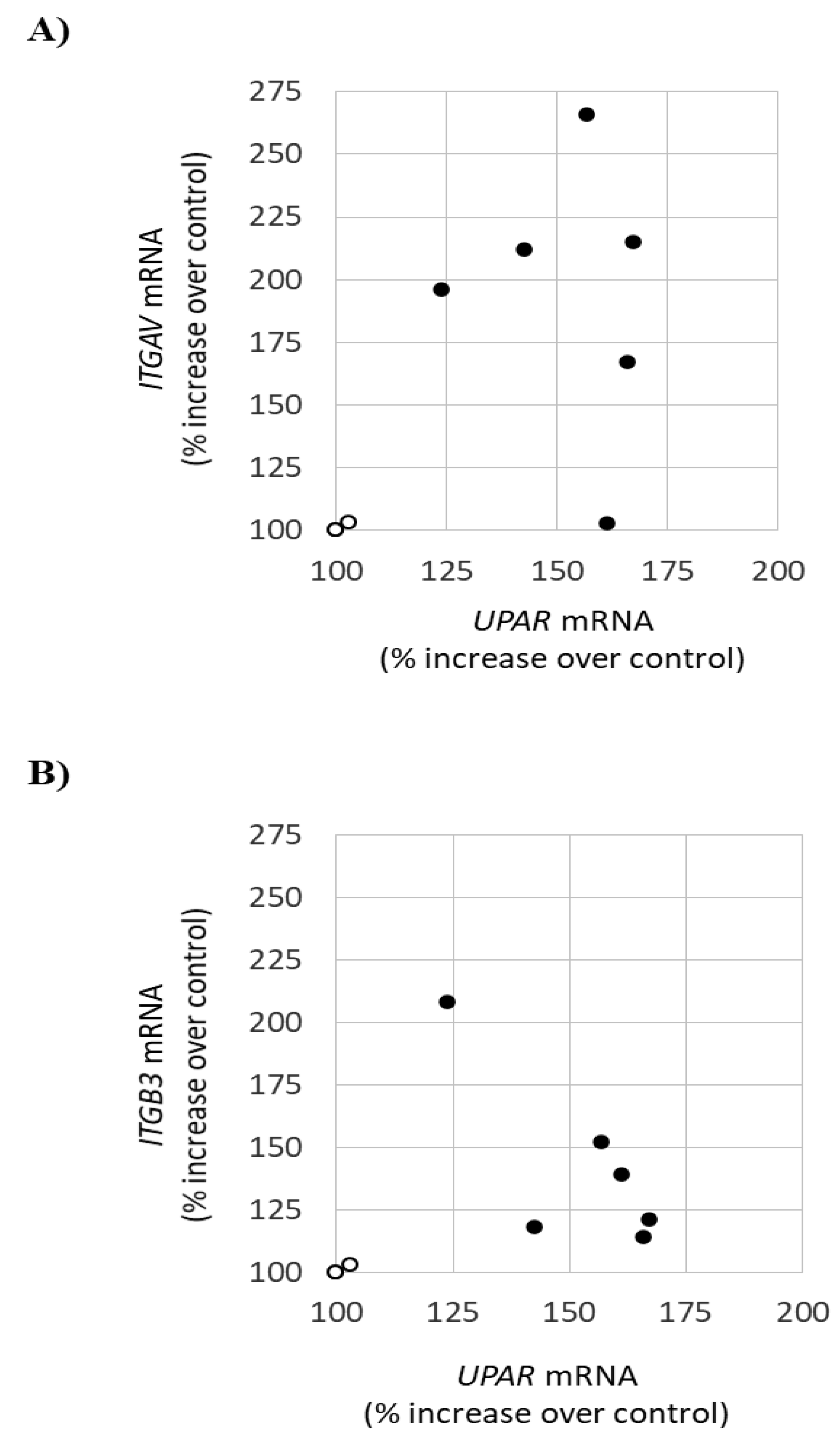
3.1. Lyso-Gb3 Increases ITGAV and ITGB3 Gene Expression in Cultured Human Podocytes
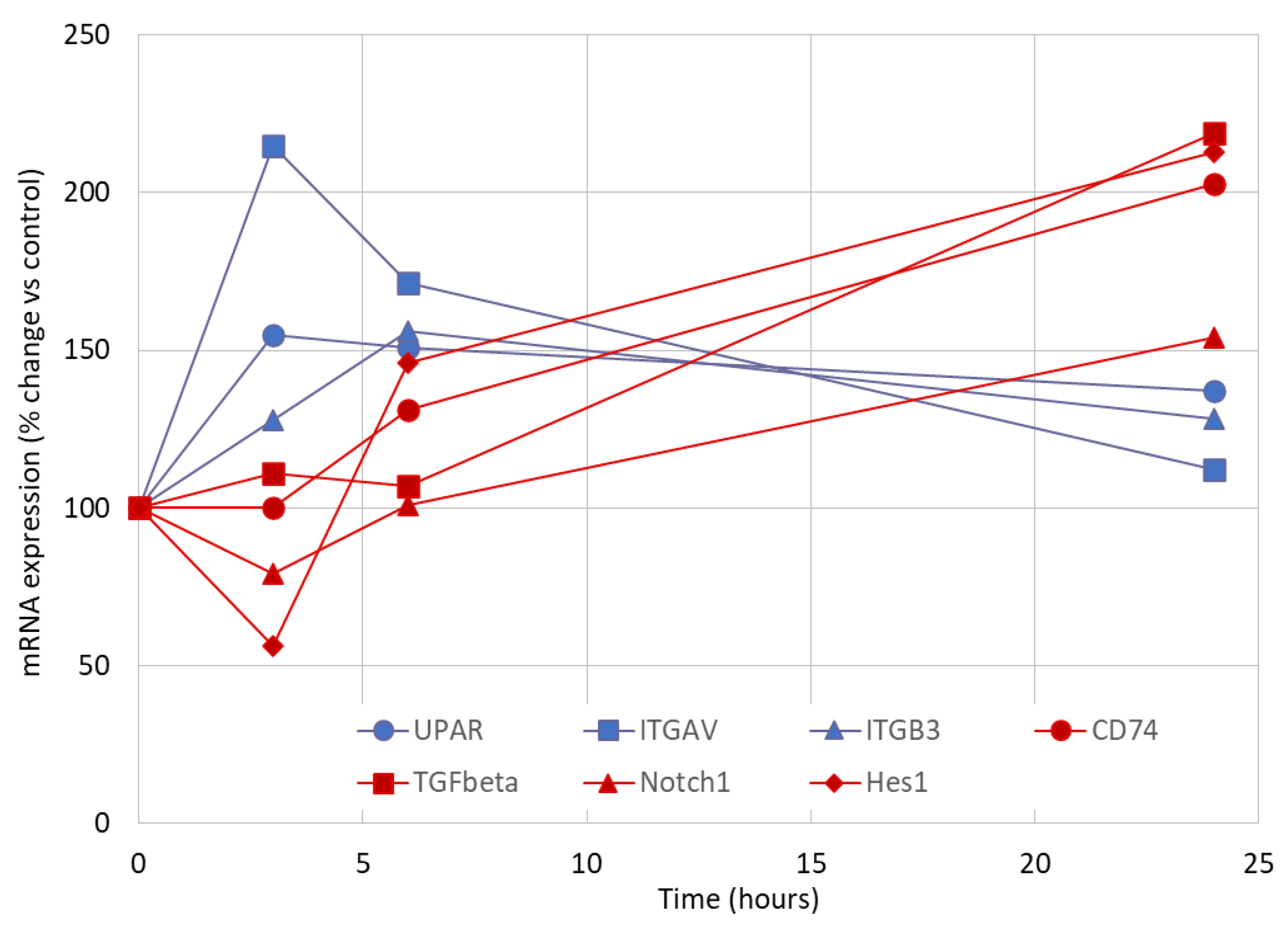
3.2. Early Markers of Lyso-Gb3-Induced Stress in Cultured Human Podocytes
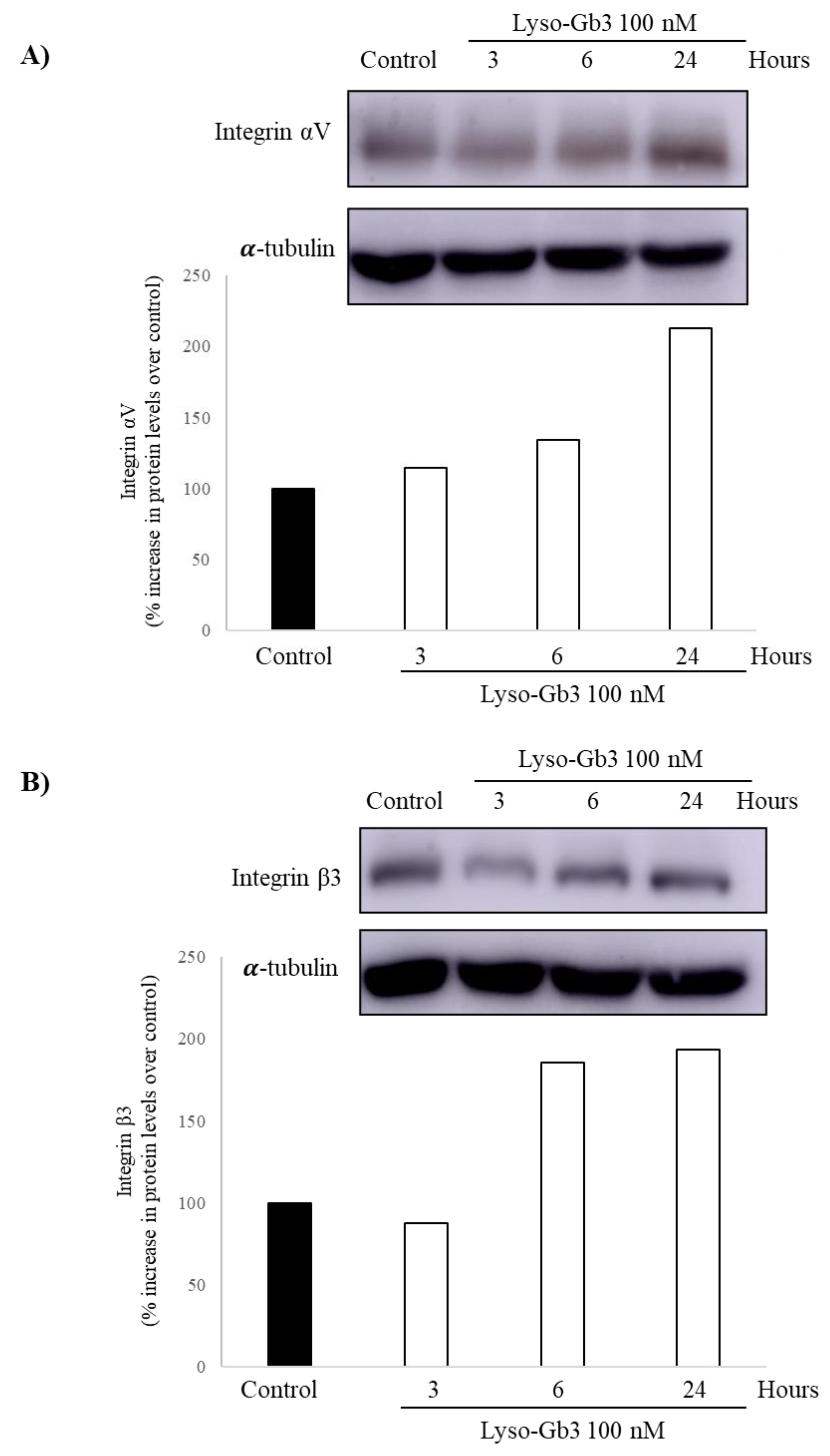
3.3. Lyso-Gb3 Increases Integrin Av and Β3 Protein in Cultured Human Podocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Statement of Ethics
References
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L. Enzymatic defect in Fabry’s disease, Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967, 276, 1163–1167. [Google Scholar]
- Kanda, A.; Nakao, S.; Tsuyama, S.; Murata, F.; Kanzaki, T. Fabry disease: Ultrastructural lectin histochemical analyses of lysosomal deposits. Virchows Arch. 2000, 436, 36–42. [Google Scholar] [PubMed]
- Askari, H.; Kaneski, C.R.; Semino-Mora, C.; Desai, P.; Ang, A.; Kleiner, D.E.; Perlee, L.T.; Quezado, M.; Spollen, L.E.; Wustman, B.A.; et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007, 451, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Zarate, Y.A.; Hopkin, R.J. Fabry’s disease. Lancet 2008, 372, 1427–1435. [Google Scholar]
- Lloyd-Evans, E.; Pelled, D.; Riebeling, C.; Bodennet, J.; de-Morgan, A.; Waller, H.; Schiffmann, R.; Futerman, A.H. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J. Biol. Chem. 2003, 278, 23594–23599. [Google Scholar] [PubMed] [Green Version]
- Pelled, D.; Trajkovic-Bodennec, S.; Lloyd-Evans, E.; Sidransky, E.; Schiffmann, R.; Futerman, A.H. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol. Dis. 2005, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Waldek, S.; Banikazemi, M.; Bushinsky, B.A.; Charrow, J.; Desnick, R.J.; Lee, P.; Loew, T.; Vedder, A.C.; Abichandani, R.; et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J. Am. Soc. Nephrol. 2007, 18, 1547–1557. [Google Scholar]
- Tøndel, C.; Bostad, L.; Larsen, K.K.; Hirth, A.; Vikse, B.E.; Houge, V.; Svarstad, E. Agalsidase benefits renal histology in young patients with Fabry disease. J. Am. Soc. Nephrol. 2013, 24, 137–148. [Google Scholar]
- Trimarchi, H. Podocyturia: What is in a name? J. Transl. Intern. Med. 2015, 3, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Cianciaruso, B.; Cizmarik, M.; Germain, D.P.; Mignani, R.; Oliveira, J.P.; Villalobos, J.; Vujkovac, B.; Waldek, S.; Wanner, C.; et al. End-stage renal disease in patients with Fabry disease: Natural history data from the Fabry Registry. Nephrol. Dial. Transplant. 2010, 25, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Politei, J.; Stern, A.; Andrews, J.; Paulero, M.; Rengel, T.; Aráoz, A.; Forrester, M.; et al. Podocyturia is significantly elevated in untreated vs treated Fabry adult patients. J. Nephrol. 2016, 6, 791–797. [Google Scholar]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A.; Wei, Y. Protease crosstalk with integrins: The urokinase receptor paradigm. Thromb. Haemost. 2001, 86, 124–129. [Google Scholar] [PubMed]
- Wei, C.; Möller, C.C.; Altintas, M.M.; Li, J.; Schwarz, K.; Zacchigna, S.; Xie, L.; Henger, A.; Schmid, H.; Rastaldi, M.P.; et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 2008, 14, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Politei, J.; Costales-Collaguazo, C.; Stern, A.; Paulero, M.; Rengel, T.; Valiño-Rivas, L.; Forrester, M.; et al. Expression of uPAR in Urinary Podocytes of Patients with Fabry Disease. Int. J. Nephrol. 2017, 2017, 1287289. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized Human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar]
- Sanchez-Niño, M.D.; Carpio, D.; Sanz, A.B.; Ruiz-Ortega, M.; Mezzano, S.; Ortiz, A. Lyso-Gb3 activates Notch1 in human podocytes. Hum. Mol. Genet. 2015, 24, 5720–5732. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Niño, M.D.; Sanz, A.B.; Carrasco, S.; Saleem, M.A.; Mathieson, P.W.; Valdivielso, J.M.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Globotriaosylsphingosine actions on human glomerular podocytes: Implications for Fabry nephropathy. Nephrol. Dial. Transplant. 2011, 26, 1797–1802. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Niño, M.D.; Sanz, A.B.; Ihalmo, P.; Lassila, M.; Hothofer, H.; Mezzano, S.; Aros, C.; Groop, P.H.; Saleem, M.A.; Mathieson, P.W.; et al. The MIF receptor CD74 in diabetic podocyte injury. J. Am. Soc. Nephrol. 2009, 20, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Niño, M.D.; Sanz, A.B.; Sanchez-López, E.; Ruiz-Ortgea, M.; Benito-Martín, A.; Saleem, M.A.; Mathieson, P.W.; Mezzano, S.; Egido, J.; Ortiz, A. HSP27/HSPB1 as an adaptive podocyte antiapoptotic protein activated by high glucose and angiotensin II. Lab. Investig. 2012, 92, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Niño, M.D.; Poveda, J.; Carrasco, S.; Ruiz-Ortega, M.; Selgas, R.; Egido, J.; Ortiz, A. 3,4-DGE is cytotoxic and decreases HSP27/HSPB1 in podocytes. Arch. Toxicol. 2014, 88, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Navarro, A.; Sanchez-Niño, M.D.; Guerrero-Hue, M.; García-Caballero, C.; Gutiérrez, E.; Yuste, C.; Sevillano, A.; Praga, M.; Egea, J.; Román, E.; et al. Podocytes are new cellular targets of haemoglobin-mediated renal damage. J. Pathol. 2018, 244, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Cuarental, L.; Valiño-Rivas, L.; Mendonça, L.; Saleem, M.; Mezzano, S.; Sanz, A.B.; Ortiz, A.; Sánchez-Niño, M.D. Tacrolimus Prevents TWEAK-Induced PLA2R Expression in Cultured Human Podocytes. J. Clin. Med. 2020, 9, 2178. [Google Scholar] [CrossRef]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Costales-Collaguazo, C.; Politei, J.; Stern, A.; Andrews, J.; Paulero, M.; Rengel, T.; Forrester, M.; et al. Increased urinary CD80 excretion and podocyturia in Fabry disease. J. Transl. Med. 2016, 14, 289. [Google Scholar] [CrossRef] [Green Version]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Fernandez, B.; Izquierdo, M.C.; Valiño-Rivas, L.; Nastou, D.; Sanz, A.B.; Ortiz, A.; Sánchez-Niño, M.D. Albumin downregulates Klotho in tubular cells. Nephrol. Dial. Transplant. 2018, 33, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Najafian, B.; Svarstad, E.; Bostad, L.; Gubler, M.C.; Tondell, C.; Whitley, C.; Mauer, M. Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int. 2011, 79, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Oliveira, J.P.; Waldek, S.; Warnock, D.G.; Cianciaruso, B.; Wanner, C. Nephropathy in males and females with Fabry disease: Cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol. Dial. Transplant. 2008, 23, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Germain, D.P.; Charrow, J.; Desnick, R.J.; Guffon, N.; Kempf, J.; Lachmann, R.H.; Lernay, R.; Linthorst, J.E.; Packman, S.; Scott, R.C.; et al. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J. Med. Genet. 2015, 52, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.G.; Ortiz, A.; Mauer, M.; Linthorst, G.; Oliveira, J.P.; Serra, A.L.; Marodi, L.; Mignani, R.; Vujkovac, B.; Beitner-Johnson, D.; et al. Renal outcomes of agalsidase beta treatment for Fabry disease: Role of proteinuria and timing of treatment initiation. Nephrol. Dial. Transplant. 2012, 27, 1042–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, C.; Oliveira, J.P.; Ortiz, A.; Mauer, M.; Germain, D.P.; Linthorst, G.E.; Serra, A.L.; Marodi, L.; Mignani, R.; Cianciaruso, B.; et al. Prognostic indicators of renal disease progression in adults with Fabry disease: Natural history data from the Fabry Registry. Clin. J. Am. Soc. Nephrol. 2010, 5, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Integrins. J. Clin. Investig. 1991, 87, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adler, S. Integrin matrix receptors in renal injury. Kidney Int. Suppl. 1994, 45, S86. [Google Scholar] [PubMed]
- Utsumi, K.; Itoh, K.; Kase, R.; Shimmoto, M.; Yamamoto, M.; Katagiri, Y.; Tanoue, K.; Kotani, M.; Ozawa, T.; Oguchi, T.; et al. Urinary excretion of the vitronectin receptor (integrin alpha V beta 3) in patients with Fabry disease. Clin. Chim. Acta 1999, 279, 55–68. [Google Scholar] [CrossRef]
- Fornoni, A.; Merscher, S.; Kopp, J.B. Lipid biology of the podocyte—New perspectives offer new opportunities. Nat. Rev. Nephrol. 2014, 10, 379–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preissner, K.T.; Kanse, S.M.; May, A.E. Urokinase receptor: A molecular organizer in cellular communication. Curr. Opin. Cell Biol. 2000, 12, 621–628. [Google Scholar] [CrossRef]
- Sachs, N.; Sonnenberg, A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013, 9, 200–210. [Google Scholar] [CrossRef]
- Brown, E.J. Integrin-associated proteins. Curr. Opin. Cell Biol. 2002, 14, 603–607. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, S.; Shi, W.; Yan, Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol. Dial. Transplant. 2012, 27, 1746–1755. [Google Scholar] [CrossRef] [Green Version]
- Trimarchi, H.; Forrester, M.; Lombi, F.; Pomeranz, V.; Raña, S.; Karl, A.; Andrews, J. Amiloride as an Alternate Adjuvant Antiproteinuric Agent in Fabry Disease: The Potential Roles of Plasmin and uPAR. Case Rep. Nephrol. 2014, 2014, 854521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.-C.; Huang, Y.-H.; Chen, Y.-J.; Kao, S.M.; Lin, H.Y.; Huang, C.K.; Liu, H.C.; Hsu, T.R.; Lin, S.P.; Yang, C.F.; et al. Plasma globotriaosylsphingosine (lysoGb3) could be a biomarker for Fabry disease with a Chinese hotspot late-onset mutation (IVS4+919G>A). Clin. Chim. Acta 2013, 426, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, D.P.; Brand, E.; Burlina, A.; Cecchi, F.; Garman, S.C.; Kempf, J.; Laney, D.A.; Linhart, A.; Marodi, L.; Nicholls, K.; et al. Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: A multicenter Fabry Registry study. Mol. Genet. Genom. Med. 2018, 6, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meehan, S.M.; Junsanto, T.; Rydell, J.J.; Desnick, R.J. Fabry disease: Renal involvement limited to podocyte pathology and proteinuria in a septuagenarian cardiac variant. Pathologic and therapeutic implications. Am. J. Kidney Dis. 2004, 43, 164–171. [Google Scholar] [CrossRef]
- Sanchez-Niño, M.D.; Ortiz, A. Notch3 and kidney injury: Never two without three. J. Pathol. 2012, 228, 266–273. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, J.; Wang, H.; Allgayer, H.; Murrell, G.A.; Boyd, D. Identification of a novel nuclear factor-kappaB sequence involved in expression of urokinase-type plasminogen activator receptor. Eur. J. Biochem. 2000, 267, 3248–3254. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.S.; Chang, H.J.; Baek, M.K.; Kim, H.R.; Shin, B.A.; Ahn, B.W.; Jun, Y.D. Lysophosphatidic acid promotes cell invasion by up-regulating the urokinase-type plasminogen activator receptor in human gastric cancer cells. J. Cell Biochem. 2008, 104, 1102–1112. [Google Scholar] [CrossRef]
- Bin Hafeez, B.; Adhami, V.M.; Asim, M.; Siddiqui, I.A.; Bhat, K.M.; Zhong, W.; Saleem, M.; Din, M.; Setaluri, V.; Mukhtar, H. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clin. Cancer Res. 2009, 15, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Raghu, H.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch-1 receptor. Mol. Cancer 2011, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Scatena, M.; Giachelli, C. The alpha(v)beta3 integrin, NF-kappaB, osteoprotegerin endothelial cell survival pathway. Potential role in angiogenesis. Trends Cardiovasc. Med. 2002, 12, 83–88. [Google Scholar] [CrossRef]
- Skrunes, R.; Tøndel, C.; Leh, S.; Larsen, K.K.; Houge, G.; Davidsen, E.S.; Hollak, C.; van Kuilenburg, A.B.; Vaz, F.M.; Svarstad, E. Long-Term Dose-Dependent Agalsidase Effects on Kidney Histology in Fabry Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1470–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimarchi, H.; Ortiz, A.; Sánchez-Niño, M.D. Lyso-Gb3 Increases αvβ3 Integrin Gene Expression in Cultured Human Podocytes in Fabry Nephropathy. J. Clin. Med. 2020, 9, 3659. https://doi.org/10.3390/jcm9113659
Trimarchi H, Ortiz A, Sánchez-Niño MD. Lyso-Gb3 Increases αvβ3 Integrin Gene Expression in Cultured Human Podocytes in Fabry Nephropathy. Journal of Clinical Medicine. 2020; 9(11):3659. https://doi.org/10.3390/jcm9113659
Chicago/Turabian StyleTrimarchi, Hernán, Alberto Ortiz, and Maria Dolores Sánchez-Niño. 2020. "Lyso-Gb3 Increases αvβ3 Integrin Gene Expression in Cultured Human Podocytes in Fabry Nephropathy" Journal of Clinical Medicine 9, no. 11: 3659. https://doi.org/10.3390/jcm9113659




