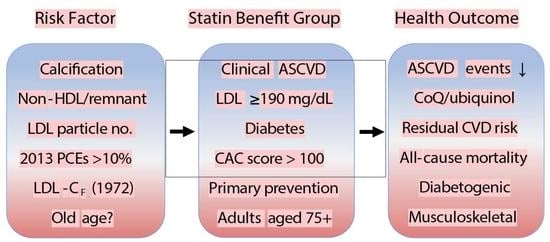
Statin Treatment in Specific Patient Groups: Role for Improved Cardiovascular Risk Markers
Abstract
1. Introduction
2. Potential for Adverse Effects
3. Cholesterol Levels and Clinical Outcomes
3.1. Low-Density Lipoprotein and Total Cholesterol
3.2. Lipoprotein Subfractions
4. Accuracy of Cardiovascular Risk Calculators: The Pooled Cohort Equations
5. Methodology
6. Efficacy of Statin Treatment in the Elderly
6.1. Primary Prevention of ASCVD
6.2. Primary Prevention with Diabetes
6.3. Secondary Prevention of ASCVD
7. Coronary Artery Calcification for Guiding Treatment Decisions
8. Coenzyme Q and Muscle Function: Role for Supplementation
9. Primary Prevention in Middle-Age Adults
10. Effect of Lipid-Lowering Medication on Lipoprotein Subfractions
11. Perspective
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Heart Disease and Stroke. Available online: www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm (accessed on 2 August 2020).
- Byrne, P.; Cullinan, J.; Smith, S.M. Statins for primary prevention of cardiovascular disease. BMJ 2019, 367, l5674. [Google Scholar] [CrossRef]
- Salami, J.A.; Warraich, H.; Valero-Elizondo, J.; Spatz, E.S.; Desai, N.R.; Rana, J.S.; Virani, S.S.; Blankstein, R.; Khera, A.; Blaha, M.J.; et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: Insights from the medical expenditure panel survey. JAMA Cardiol. 2017, 2, 56–65. [Google Scholar] [CrossRef]
- Curfman, G. Risks of statin therapy in older adults. JAMA Intern. Med. 2017, 177, 966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nayor, M.; Vasan, R.S. Recent update to the US cholesterol treatment guidelines: A comparison with international guidelines. Circulation 2016, 133, 1795–1806. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Falk, E. Primary prevention with statins in the elderly. J. Am. Coll. Cardiol. 2018, 71, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J. Statins in persons at low risk of cardiovascular disease. Am. Fam. Physician 2017, 96. Online. [Google Scholar]
- Leya, M.; Stone, N.J. Statin prescribing in the elderly: Special considerations. Curr. Atheroscler. Rep. 2017, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef]
- Byrne, P.; Cullinan, J.; Smith, A.; Smith, S.M. Statins for the primary prevention of cardiovascular disease: An overview of systematic reviews. BMJ Open 2019, 9, e023085. [Google Scholar] [CrossRef]
- Yebyo, H.G.; Aschmann, H.E.; Puhan, M.A. Finding the balance between benefits and harms when using statins for primary prevention of cardiovascular disease: A modeling study. Ann. Intern. Med. 2019, 170, 1–10. [Google Scholar] [CrossRef]
- Pencina, M.J.; Navar-Boggan, A.M.; D’Agostino, R.B., Sr.; Williams, K.; Neely, B.; Sniderman, A.D.; Peterson, E.D. Application of new cholesterol guidelines to a population-based sample. N. Engl. J. Med. 2014, 370, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Buettner, C.; Davis, R.B.; Leveille, S.G.; Mittleman, M.A.; Mukamal, K.J. Prevalence of musculoskeletal pain and statin use. J. Gen. Intern. Med. 2008, 23, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Millan Nunez-Cortes, J.; Chillaron, J.J.; Flores-Le Roux, J.A.; Rius, J. Severity of statin-induced adverse effects on muscle and associated conditions: Data from the DAMA study. Expert Opin. Drug Saf. 2016, 15, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Dresser, G.K. Overcoming challenges with statin therapy. J. Am. Heart Assoc. 2016, 5, e002497. [Google Scholar] [CrossRef]
- Abramson, J.D.; Rosenberg, H.G.; Jewell, N.; Wright, J.M. Should people at low risk of cardiovascular disease take a statin? BMJ 2013, 347, f6123. [Google Scholar] [CrossRef]
- Mansi, I.; Frei, C.R.; Pugh, M.J.; Makris, U.; Mortensen, E.M. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern. Med. 2013, 173, 1–10. [Google Scholar] [CrossRef]
- Macedo, A.F.; Taylor, F.C.; Casas, J.P.; Adler, A.; Prieto-Merino, D.; Ebrahim, S. Unintended effects of statins from observational studies in the general population: Systematic review and meta-analysis. BMC Med. 2014, 12, 51. [Google Scholar] [CrossRef]
- Makris, U.E.; Alvarez, C.A.; Wei, W.; Mortensen, E.M.; Mansi, I.A. Association of statin use with risk of back disorder diagnoses. JAMA Intern. Med. 2017, 177, 1044–1046. [Google Scholar] [CrossRef]
- Brailovski, E.; Kim, R.B.; Juurlink, D. Rosuvastatin myotoxicity after starting canagliflozin treatment: A case report. Ann. Intern. Med. 2020, 173, 585–587. [Google Scholar] [CrossRef]
- Strandberg, T.E. Role of statin therapy in primary prevention of cardiovascular disease in elderly patients. Curr. Atheroscler. Rep. 2019, 21, 28. [Google Scholar] [CrossRef]
- Leutner, M.; Matzhold, C.; Bellach, L.; Deischinger, C.; Harreiter, J.; Thurner, S.; Klimek, P.; Kautzky-Willer, A. Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann. Rheum. Dis. 2019, 78, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Tangelloju, S.; Little, B.B.; Esterhay, R.J.; Brock, G.; LaJoie, S. Statins are associated with new onset type 2 diabetes mellitus (T2DM) in Medicare patients >/=65 years. Diabetes Metab. Res. Rev. 2020, 36, e3310. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.A.; Gomes, T.; Camacho, X.; Juurlink, D.N.; Shah, B.R.; Mamdani, M.M. Risk of incident diabetes among patients treated with statins: Population based study. BMJ 2013, 346, f2610. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Carmena, R.; Betteridge, D.J. Diabetogenic action of statins: Mechanisms. Curr. Atheroscler. Rep. 2019, 21, 23. [Google Scholar] [CrossRef]
- Casula, M.; Mozzanica, F.; Scotti, L.; Tragni, E.; Pirillo, A.; Corrao, G.; Catapano, A.L. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 396–406. [Google Scholar] [CrossRef]
- Rees-Milton, K.J.; Norman, P.; Babiolakis, C.; Hulbert, M.; Turner, M.E.; Berger, C.; Anastassiades, T.P.; Hopman, W.M.; Adams, M.A.; Powley, W.L.; et al. Statin use is associated with insulin resistance in participants of the Canadian Multicentre Osteoporosis Study. J. Endocr. Soc. 2020, 4, bvaa057. [Google Scholar] [CrossRef]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef]
- Tikhonoff, V.; Casiglia, E.; Mazza, A.; Scarpa, R.; Thijs, L.; Pessina, A.C.; Staessen, J.A. Low-density lipoprotein cholesterol and mortality in older people. J. Am. Geriatr. Soc. 2005, 53, 2159–2164. [Google Scholar] [CrossRef]
- Jeong, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Son, J.S.; Yun, J.M.; Park, S.M. Association of change in total cholesterol level with mortality: A population-based study. PLoS ONE 2018, 13, e0196030. [Google Scholar] [CrossRef]
- Charlton, J.; Ravindrarajah, R.; Hamada, S.; Jackson, S.H.; Gulliford, M.C. Trajectory of total cholesterol in the last years of life over age 80 years: Cohort study of 99,758 participants. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Gulliford, M.C. Mortality in individuals aged 80 and older with type 2 diabetes mellitus in relation to glycosylated hemoglobin, blood pressure, and total cholesterol. J. Am. Geriatr. Soc. 2016, 64, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Tybjaerg-Hansen, A.; Stender, S.; Frikke-Schmidt, R.; Nordestgaard, B.G. Low-density lipoprotein cholesterol and the risk of cancer: A mendelian randomization study. J. Natl. Cancer Inst. 2011, 103, 508–519. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B.; Toth, P.P.; Kwiterovich, P.O.; Blumenthal, R.S.; Jones, S.R. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013, 310, 2061–2068. [Google Scholar] [CrossRef]
- Sathiyakumar, V.; Park, J.; Golozar, A.; Lazo, M.; Quispe, R.; Guallar, E.; Blumenthal, R.S.; Jones, S.R.; Martin, S.S. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation 2018, 137, 10–19. [Google Scholar] [CrossRef]
- Kastelein, J.J.; van der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef]
- Anderson, T.J.; Gregoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. 2016 Canadian Cardiovascular Society Guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef]
- Mora, S.; Caulfield, M.P.; Wohlgemuth, J.; Chen, Z.; Superko, H.R.; Rowland, C.M.; Glynn, R.J.; Ridker, P.M.; Krauss, R.M. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circulation 2015, 132, 2220–2229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: An analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Neeland, I.J.; Das, S.R.; Khera, A.; Turer, A.T.; Ayers, C.R.; McGuire, D.K.; Rohatgi, A. Relation of Black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am. J. Cardiol. 2015, 115, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, D.; Louie, J.Z.; Caulfield, M.P.; Nilsson, P.M.; Devlin, J.J.; Melander, O. LDL subfractions are associated with incident cardiovascular disease in the Malmo Prevention Project Study. Atherosclerosis 2017, 263, 287–292. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Allan, G.M.; Nouri, F.; Korownyk, C.; Kolber, M.R.; Vandermeer, B.; McCormack, J. Variation among cardiovascular risk calculators in relative risk increases with identical risk factor increases. BMC Res. Notes 2015, 8, 417. [Google Scholar] [CrossRef][Green Version]
- Damen, J.A.; Hooft, L.; Schuit, E.; Debray, T.P.; Collins, G.S.; Tzoulaki, I.; Lassale, C.M.; Siontis, G.C.; Chiocchia, V.; Roberts, C.; et al. Prediction models for cardiovascular disease risk in the general population: Systematic review. BMJ 2016, 353, i2416. [Google Scholar] [CrossRef]
- Nissen, S.E. Prevention guidelines: Bad process, bad outcome. JAMA Intern. Med. 2014, 174, 1972–1973. [Google Scholar] [CrossRef]
- Preiss, D.; Kristensen, S.L. The new pooled cohort equations risk calculator. Can. J. Cardiol. 2015, 31, 613–619. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Ridker, P.M. Further insight into the cardiovascular risk calculator: The roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern. Med. 2014, 174, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Ridker, P.M. Calibration of the pooled cohort equations for atherosclerotic cardiovascular disease: An update. Ann. Intern. Med. 2016, 165, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Damen, J.A.; Pajouheshnia, R.; Heus, P.; Moons, K.G.M.; Reitsma, J.B.; Scholten, R.; Hooft, L.; Debray, T.P.A. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: A systematic review and meta-analysis. BMC Med. 2019, 17, 109. [Google Scholar] [CrossRef]
- Rana, J.S.; Tabada, G.H.; Solomon, M.D.; Lo, J.C.; Jaffe, M.G.; Sung, S.H.; Ballantyne, C.M.; Go, A.S. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J. Am. Coll. Cardiol. 2016, 67, 2118–2130. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N.R. Statins: New American guidelines for prevention of cardiovascular disease. Lancet 2013, 382, 1762–1765. [Google Scholar] [CrossRef]
- Yadlowsky, S.; Hayward, R.A.; Sussman, J.B.; McClelland, R.L.; Min, Y.I.; Basu, S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann. Intern. Med. 2018, 169, 20–29. [Google Scholar] [CrossRef]
- Pylypchuk, R.; Wells, S.; Kerr, A.; Poppe, K.; Riddell, T.; Harwood, M.; Exeter, D.; Mehta, S.; Grey, C.; Wu, B.P.; et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: A derivation and validation study. Lancet 2018, 391, 1897–1907. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Go, A.S.; Fortmann, S.P. Statins for primary prevention in older adults: Uncertainty and the need for more evidence. JAMA 2016, 316, 1971–1972. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; Garcia, F.A.R.; Gillman, M.W.; Kemper, A.R.; Krist, A.H.; et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA 2016, 316, 1997–2007. [Google Scholar] [CrossRef]
- Mehta, S.; Jackson, R.; Poppe, K.; Kerr, A.J.; Pylypchuk, R.; Wells, S. How do cardiovascular risk prediction equations developed among 30-74 year olds perform in older age groups? A validation study in 125,000 people aged 75-89 years. J. Epidemiol. Community Health 2020, 74, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Motamed, N.; Perumal, D.; Zamani, F.; Ashrafi, H.; Haghjoo, M.; Saeedian, F.S.; Maadi, M.; Akhavan-Niaki, H.; Rabiee, B.; Asouri, M. Conicity index and waist-to-hip ratio are superior obesity indices in predicting 10-year cardiovascular risk among men and women. Clin. Cardiol. 2015, 38, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Rost, S.; Freuer, D.; Peters, A.; Thorand, B.; Holle, R.; Linseisen, J.; Meisinger, C. New indexes of body fat distribution and sex-specific risk of total and cause-specific mortality: A prospective cohort study. BMC Public Health 2018, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Petursson, H.; Sigurdsson, J.A.; Bengtsson, C.; Nilsen, T.I.; Getz, L. Is the use of cholesterol in mortality risk algorithms in clinical guidelines valid? Ten years prospective data from the Norwegian HUNT 2 study. J. Eval. Clin. Pract. 2012, 18, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Yi, J.J.; Ohrr, H. Total cholesterol and all-cause mortality by sex and age: A prospective cohort study among 12.8 million adults. Sci. Rep. 2019, 9, 1596. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017, 17, 196. [Google Scholar] [CrossRef]
- Ramos, R.; Comas-Cufi, M.; Marti-Lluch, R.; Ballo, E.; Ponjoan, A.; Alves-Cabratosa, L.; Blanch, J.; Marrugat, J.; Elosua, R.; Grau, M.; et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: Retrospective cohort study. BMJ 2018, 362, k3359. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Comas-Cufi, M.; Blanch, J.; Marti, R.; Ponjoan, A.; Alves-Cabratosa, L.; Petersen, I.; Marrugat, J.; Elosua, R.; Grau, M.; et al. Effectiveness of statins as primary prevention in people with different cardiovascular risk: A population-based cohort study. Clin. Pharmacol. Ther. 2018, 104, 719–732. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Fergestrom, N.; Gage, B.F.; Paisley, R.; Moon, P.; Novak, E.; Cheezum, M.; Shaw, L.J.; Villines, T.C. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J. Am. Coll. Cardiol. 2018, 72, 3233–3242. [Google Scholar] [CrossRef]
- Ponce, O.J.; Larrea-Mantilla, L.; Hemmingsen, B.; Serrano, V.; Rodriguez-Gutierrez, R.; Spencer-Bonilla, G.; Alvarez-Villalobos, N.; Benkhadra, K.; Haddad, A.; Gionfriddo, M.R.; et al. Lipid-lowering agents in older individuals: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Endocrinol. Metab. 2019, 104, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Sutin, D.; Williamson, J.D.; Davis, B.R.; Piller, L.B.; Pervin, H.; Pressel, S.L.; Blaum, C.S.; Group, A.C.R. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: The ALLHAT-LLT randomized clinical trial. JAMA Intern. Med. 2017, 177, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Lonn, E.; Paynter, N.P.; Glynn, R.; Yusuf, S. Primary prevention with statin therapy in the elderly: New meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation 2017, 135, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Gotto, A.M., Jr.; Paolillo, S.; D’Amore, C.; Losco, T.; Musella, F.; Scala, O.; Marciano, C.; Ruggiero, D.; Marsico, F.; et al. Benefits of statins in elderly subjects without established cardiovascular disease: A meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 2090–2099. [Google Scholar] [CrossRef]
- Zhai, C.; Hou, K.; Li, R.; Hu, Y.; Zhang, J.; Zhang, Y.; Wang, L.; Zhang, R.; Cong, H. Efficacy of statin treatment based on cardiovascular outcomes in elderly patients: A standard meta-analysis and Bayesian network analysis. J. Int. Med. Res. 2020, 48, 300060520926349. [Google Scholar] [CrossRef]
- Orkaby, A.R.; Driver, J.A.; Ho, Y.L.; Lu, B.; Costa, L.; Honerlaw, J.; Galloway, A.; Vassy, J.L.; Forman, D.E.; Gaziano, J.M.; et al. Association of statin use with all-cause and cardiovascular mortality in US veterans 75 years and older. JAMA 2020, 324, 68–78. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.J.; Shim, C.Y.; Kim, J.S.; Kim, B.K.; Park, S.; Chang, H.J.; Hong, G.R.; Ko, Y.G.; Kang, S.M.; et al. Statin and clinical outcomes of primary prevention in individuals aged >75years: The SCOPE-75 study. Atherosclerosis 2019, 284, 31–36. [Google Scholar] [CrossRef]
- Neves, P.O.; Andrade, J.; Moncao, H. Coronary artery calcium score: Current status. Radiol. Bras. 2017, 50, 182–189. [Google Scholar] [CrossRef]
- Sarwar, A.; Shaw, L.J.; Shapiro, M.D.; Blankstein, R.; Hoffmann, U.; Cury, R.C.; Abbara, S.; Brady, T.J.; Budoff, M.J.; Blumenthal, R.S.; et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc. Imaging 2009, 2, 675–688. [Google Scholar] [CrossRef]
- Miedema, M.D.; Dardari, Z.A.; Nasir, K.; Blankstein, R.; Knickelbine, T.; Oberembt, S.; Shaw, L.; Rumberger, J.; Michos, E.D.; Rozanski, A.; et al. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw. Open 2019, 2, e197440. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Fuster, V.; Muntendam, P.; Mehran, R.; Baber, U.; Sartori, S.; Falk, E. Negative risk markers for cardiovascular events in the elderly. J. Am. Coll. Cardiol. 2019, 74, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Nakano, C.; Obi, Y.; Matsui, I.; Kusunoki, Y.; Mori, D.; Oka, T.; Hashimoto, N.; Takabatake, Y.; et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS ONE 2016, 11, e0163673. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.; Niemeijer, M.N.; Leening, M.J.; van den Berg, M.E.; Franco, O.H.; Deckers, J.W.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J. Am. Heart Assoc. 2016, 5, e002707. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Nordestgaard, B.G. Statin use in primary prevention of atherosclerotic cardiovascular disease according to 5 major guidelines for sensitivity, specificity, and number needed to treat. JAMA Cardiol. 2019, 4, 1131–1138. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef]
- Yano, Y.; O’Donnell, C.J.; Kuller, L.; Kavousi, M.; Erbel, R.; Ning, H.; D’Agostino, R.; Newman, A.B.; Nasir, K.; Hofman, A.; et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: An analysis of pooled population-based studies. JAMA Cardiol. 2017, 2, 986–994. [Google Scholar] [CrossRef]
- Lind, L.; Sundstrom, J.; Arnlov, J.; Lampa, E. Impact of aging on the strength of cardiovascular risk factors: A longitudinal study over 40 years. J. Am. Heart Assoc. 2018, 7, e007061. [Google Scholar] [CrossRef]
- Kalen, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef]
- Niklowitz, P.; Onur, S.; Fischer, A.; Laudes, M.; Palussen, M.; Menke, T.; Doring, F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J. Clin. Biochem. Nutr. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Schaffer, A.; Cicurel, A.; Feldhamer, I.; Tal, A.; Cheng, K.; Sinha, S.; Schiff, E.; Lavie, G.; Ruppin, E. Large population study identifies drugs associated with reduced COVID-19 severity. medRxiv 2020. [Google Scholar] [CrossRef]
- McMurray, J.J.; Dunselman, P.; Wedel, H.; Cleland, J.G.; Lindberg, M.; Hjalmarson, A.; Kjekshus, J.; Waagstein, F.; Apetrei, E.; Barrios, V.; et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: A pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J. Am. Coll. Cardiol. 2010, 56, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.L.; Rosenfeldt, F.; Filipiak, K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019, 26, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Investigators, Q.S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Serban, C.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; Watts, G.F.; et al. Statin therapy and plasma coenzyme Q10 concentrations--A systematic review and meta-analysis of placebo-controlled trials. Pharmacol. Res. 2015, 99, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Stancato, A.; Aleo, E.; Dmitrieva, A.; Littarru, G.P. Statins lower plasma and lymphocyte ubiquinol/ubiquinone without affecting other antioxidants and PUFA. Biofactors 2003, 18, 113–124. [Google Scholar] [CrossRef]
- Schirris, T.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.; Brandt, U.; Koopman, W.J.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-induced myopathy Is associated with mitochondrial complex III inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Conn, C.A.; Trujillo, K.A. Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: Implications for statin-induced rhabdomyolysis. Eur. J. Pharmacol. 2013, 711, 1–9. [Google Scholar] [CrossRef]
- Turner, R.M.; Fontana, V.; FitzGerald, R.; Morris, A.P.; Pirmohamed, M. Investigating the clinical factors and comedications associated with circulating levels of atorvastatin and its major metabolites in secondary prevention. Br. J. Clin. Pharmacol. 2020, 86, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Pirmohamed, M. Statin-related myotoxicity: A comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Mount Sinai. Coenzyme Q10. Available online: www.mountsinai.org/health-library/supplement/coenzyme-q10 (accessed on 13 November 2020).
- Caso, G.; Kelly, P.; McNurlan, M.A.; Lawson, W.E. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. Am. J. Cardiol. 2007, 99, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Fedacko, J.; Pella, D.; Fedackova, P.; Hanninen, O.; Tuomainen, P.; Jarcuska, P.; Lopuchovsky, T.; Jedlickova, L.; Merkovska, L.; Littarru, G.P. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can. J. Physiol. Pharmacol. 2013, 91, 165–170. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Wada, H.; Goto, H.; Hagiwara, S.; Yamamoto, Y. Redox status of coenzyme Q10 is associated with chronological age. J. Am. Geriatr. Soc. 2007, 55, 1141–1142. [Google Scholar] [CrossRef]
- Evans, M.; Baisley, J.; Barss, S.; Guthrie, N. A randomized, double-blind trial on the bioavailability of two CoQ10 formulations. J. Funct. Food. 2009, 1, 65–732. [Google Scholar] [CrossRef]
- Langsjoen, P.H.; Langsjoen, A.M. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors 2008, 32, 119–128. [Google Scholar] [CrossRef]
- Langsjoen, P.H.; Langsjoen, A.M. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin. Pharmacol. Drug Dev. 2013, 3, 13–17. [Google Scholar] [CrossRef]
- Xia, L.; Nordman, T.; Olsson, J.M.; Damdimopoulos, A.; Bjorkhem-Bergman, L.; Nalvarte, I.; Eriksson, L.C.; Arner, E.S.; Spyrou, G.; Bjornstedt, M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone: A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003, 278, 2141–2146. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Dahlstrom, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.; Johansson, P. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: Follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens. PLoS ONE 2015, 10, e0141641. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.A.; Lorson, L.; White, C.M.; Thompson, P.D. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis 2015, 238, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, A.; Neely, D.; Armitage, J.; Chinoy, H.; Cooper, R.G.; Laaksonen, R.; Carr, D.F.; Bloch, K.M.; Fahy, J.; Hanson, A.; et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 2014, 96, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kolodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.; Erqou, S.; Sever, P.; Jukema, J.W.; Ford, I.; Sattar, N. Statins and all-cause mortality in high-risk primary prevention: A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 2010, 170, 1024–1031. [Google Scholar] [CrossRef]
- Mills, E.J.; Rachlis, B.; Wu, P.; Devereaux, P.J.; Arora, P.; Perri, D. Primary prevention of cardiovascular mortality and events with statin treatments: A network meta-analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 2008, 52, 1769–1781. [Google Scholar] [CrossRef]
- Schleyer, T.; Hui, S.; Wang, J.; Zhang, Z.; Knapp, K.; Baker, J.; Chase, M.; Boggs, R.; Simpson, R.J., Jr. Quantifying unmet need in statin-treated hyperlipidemia patients and the potential benefit of further LDL-C reduction through an EHR-based retrospective cohort study. J. Manag. Care Spec. Pharm. 2019, 25, 544–554. [Google Scholar] [CrossRef]
- Kostner, K.M.; Kostner, G.M. Lipoprotein (a): A historical appraisal. J. Lipid Res. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Willeit, P.; Ridker, P.M.; Nestel, P.J.; Simes, J.; Tonkin, A.M.; Pedersen, T.R.; Schwartz, G.G.; Olsson, A.G.; Colhoun, H.M.; Kronenberg, F.; et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: Individual patient-data meta-analysis of statin outcome trials. Lancet 2018, 392, 1311–1320. [Google Scholar] [CrossRef]
- Raal, F.J.; Giugliano, R.P.; Sabatine, M.S.; Koren, M.J.; Blom, D.; Seidah, N.G.; Honarpour, N.; Lira, A.; Xue, A.; Chiruvolu, P.; et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 2016, 57, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.U.; Seo, H.S.; Lee, E.M.; Shin, S.Y.; Choi, U.J.; Na, J.O.; Lim, H.E.; Kim, J.W.; Kim, E.J.; Rha, S.W.; et al. Statins do not decrease small, dense low-density lipoprotein. Tex. Heart Inst. J. 2010, 37, 421–428. [Google Scholar] [PubMed]
- Tsimikas, S.; Gordts, P.; Nora, C.; Yeang, C.; Witztum, J.L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 2020, 41, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Balling, M.; Langsted, A.; Afzal, S.; Varbo, A.; Davey Smith, G.; Nordestgaard, B.G. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019, 286, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Wiesbauer, F.; Blessberger, H.; Demyanets, S.; Wojta, J.; Huber, K.; Maurer, G.; Schillinger, M.; Speidl, W.S. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. J. Clin. Lipidol. 2015, 9, 801–806. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Bays, H.E.; Philip, S.; Doyle, R.T., Jr.; Braeckman, R.A.; Stirtan, W.G.; Soni, P.N.; Juliano, R.A. Icosapent ethyl (eicosapentaenoic acid ethyl ester): Effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis 2016, 253, 81–87. [Google Scholar] [CrossRef]
- Wurtz, P.; Wang, Q.; Soininen, P.; Kangas, A.J.; Fatemifar, G.; Tynkkynen, T.; Tiainen, M.; Perola, M.; Tillin, T.; Hughes, A.D.; et al. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J. Am. Coll. Cardiol. 2016, 67, 1200–1210. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Abt, M.; Bao, W.; DeMicco, D.; Kallend, D.; Miller, M.; Mundl, H.; Olsson, A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015, 65, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Philip, S.; Granowitz, C.; Toth, P.P.; Wong, N.D. Residual hypertriglyceridemia and estimated atherosclerotic cardiovascular disease risk by statin use in U.S. adults with diabetes: National Health and Nutrition Examination Survey 2007–2014. Diabetes Care 2019, 42, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Tsugawa, Y.; Tseng, C.H.; Kobayashi, Y.; Shapiro, M.F. Different time trends of caloric and fat intake between statin users and nonusers among US adults: Gluttony in the time of statins? JAMA Intern. Med. 2014, 174, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Hu, E.A.; Steffen, L.M.; Coresh, J.; Appel, L.J.; Rebholz, C.M. Adherence to the Healthy Eating Index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J. Nutr. 2020, 150, 312–321. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Moli-sani Study Investigators. Interaction between Mediterranean diet and statins on mortality risk in patients with cardiovascular disease: Findings from the Moli-sani Study. Int. J. Cardiol. 2019, 276, 248–254. [Google Scholar] [CrossRef]
- Ramo, J.T.; Ripatti, P.; Tabassum, R.; Soderlund, S.; Matikainen, N.; Gerl, M.J.; Klose, C.; Surma, M.A.; Stitziel, N.O.; Havulinna, A.S.; et al. Coronary artery disease risk and lipidomic profiles are similar in hyperlipidemias with family history and population-ascertained hyperlipidemias. J. Am. Heart Assoc. 2019, 8, e012415. [Google Scholar] [CrossRef]
- Van der Ploeg, M.A.; Floriani, C.; Achterberg, W.P.; Bogaerts, J.M.K.; Gussekloo, J.; Mooijaart, S.P.; Streit, S.; Poortvliet, R.K.E.; Drewes, Y.M. Recommendations for (discontinuation of) statin treatment in older adults: Review of guidelines. J. Am. Geriatr. Soc. 2020, 68, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tricoci, P.; Allen, J.M.; Kramer, J.M.; Califf, R.M.; Smith, S.C., Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009, 301, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Giral, P.; Neumann, A.; Weill, A.; Coste, J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: A nationwide population-based cohort study in France. Eur. Heart J. 2019, 40, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
| Study/Design | Population/Size | Intervention/Outcome Assessed | Major Findings |
|---|---|---|---|
| Emerging Risk Factors Collaboration, 2009 [41] 68 long-term prospective studies from ERFC with complete data. | 302,430 adults in 21 countries (Europe and North America) with no ASCVD at baseline. 2.79 million person-years | Prediction of CHD events (myocardial infarction, stroke, or CHD deaths): adjusted HRs for 1-standard deviation higher baseline values. | Non-HDL and HDL were log-linear risk factors, but triglycerides were not an independent risk factor after adjustment (HRs: 1.50, 0.78 and 0.99). |
| Ravnskov, 2016 [29] Meta-analysis | 19 studies including 30 cohorts with a total of 68,094 adults age 60 and over. | Association of baseline LDL-C with all-cause mortality (irrespective of statin treatment). | Mortality decreased with increasing LDL-C quartile in 92% of participants (mean Q4 HR: 0.54). |
| Lei, 2017 [68] Meta-analysis | 14 randomized trials involving 2149 patients with heart failure. | Effect of coenzyme Q supplementation on heart failure outcomes. | Coenzyme Q decreased mortality (RR: 0.69, 95% CI: 0.50–0.95). |
| Ramos, 2018 [69] Retrospective cohort: SIDIAP database; new-user design | 46,864 new and non-statin users in Spain aged 75 and older with no history of ASCVD. | New statin use stratified by diabetes and two age groups: 75–84, 85+; mortality/ASCVD events. | Statins reduced ASCVD in diabetes patients (HRs: 0.76, 0.82). No ASCVD reduction in patients without diabetes (HRs: 0.94, 1.00). |
| Garcia-Gil, 2018 [70] Retrospective cohort: SIDIAP database | 617,850 primary prevention patients in Spain aged 35–74 (new users, 80% with moderate intensity statin). | ASCVD events (myocardial infarction, ischemic stroke) and all-cause mortality stratified by 10-year CHD risk categories. | 5-year NNTs for ASCVD: 470, 204, 75, and 62 for <5%, 5–7.5%, 7.5–10%, and 10–20% risk categories. |
| Mitchell, 2018 [71] Retrospective cohort | 13,644 military subjects over 18 with no prior ASCVD; 9.4-year median follow-up. | Effect of statin use versus non-use on first MACE, stratified across six CAC patient groups. | HRs for CAC = 0, 1–100, 101–400, and >400 were 1.0, 0.83, 0.32, and 0.56 (statin use vs. non-use). |
| Yi, 2019 [67] Prospective cohort: KOMERIT: Korean Metabolic Risk Factor | 12.8 million Korean adults; age groups: 18–34, 35–44, 45–54, 55–64, 65–74, 75–99. | Relationship between TC and all-cause mortality for 12 age-sex groups. | HR was a U-shaped function of TC in all 12 groups. HRs approached 1.0 for TC: 185–275 in elderly adults. |
| Ponce, 2019 [72] Meta-analysis | 23 randomized trials involving 60,194 adults 65 and older. | Statins for primary versus secondary prevention; all-cause and cardiovascular mortality/ASCVD events. | Strong evidence supporting statins for secondary but not primary prevention. |
| Yusuf, 2020 [64] Prospective cohort: PURE (Prospective Urban Rural Epidemiology) | 155,722 adults aged 35–70 without ASCVD followed for 9.5 years; 21 low and high-income countries across 5 continents. | Effects of 14 modifiable behavioral and metabolic risk factors on ASCVD events and all-cause mortality. | 70% of ASCVD events and deaths were attributable to the 14 risk factors, especially hypertension (22%) and high non-HDL-C (8%). |
| Cardiovascular Risk Factors | Contraindications/Adverse Effects |
|---|---|
| Prior history of ASCVD: statins reduce mortality and ASCVD in secondary prevention [72,76]. | Lack of benefit in primary prevention over age 75 without diabetes [69,72]. Derisk at age 65 if no risk factors [6,62]. |
| CAC = 0 and ≤10 are negative risk markers in old and young adults [81,82]. CAC > 100 may be considered a statin benefit group: 10-year NNT = 12 [71]. | Myopathy/osteoporosis/new-onset diabetes at high doses; avoid canagliflozin (see Introduction) [11]. |
| Statins modestly reduce ASCVD in primary prevention diabetes patients aged 75–84: 1-year NNT = 164 [69]. | Coenzyme Q supplementation improves health outcomes [68,107,114]. |
| Non-HDL (LDL particle number + remnant cholesterol) is more predictive than LDL-CF [64,129]. | Residual cardiovascular risk persists after LDL-C controlled by statins alone [133]. |
| 2013 pooled cohort equations overestimate 10-year ASCVD risk: increase 7.5% risk threshold to 10% for primary prevention [6,55,61]. | Healthy diet found necessary for mortality benefit of statins in secondary prevention [141]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, A.M.B.; Mishcon, H.R.; Redwanski, J.L.; Hills, R.D., Jr. Statin Treatment in Specific Patient Groups: Role for Improved Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3748. https://doi.org/10.3390/jcm9113748
White AMB, Mishcon HR, Redwanski JL, Hills RD Jr. Statin Treatment in Specific Patient Groups: Role for Improved Cardiovascular Risk Markers. Journal of Clinical Medicine. 2020; 9(11):3748. https://doi.org/10.3390/jcm9113748
Chicago/Turabian StyleWhite, Alyssa M. B., Hillary R. Mishcon, John L. Redwanski, and Ronald D. Hills, Jr. 2020. "Statin Treatment in Specific Patient Groups: Role for Improved Cardiovascular Risk Markers" Journal of Clinical Medicine 9, no. 11: 3748. https://doi.org/10.3390/jcm9113748
APA StyleWhite, A. M. B., Mishcon, H. R., Redwanski, J. L., & Hills, R. D., Jr. (2020). Statin Treatment in Specific Patient Groups: Role for Improved Cardiovascular Risk Markers. Journal of Clinical Medicine, 9(11), 3748. https://doi.org/10.3390/jcm9113748