AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. FISH Abnormalities
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
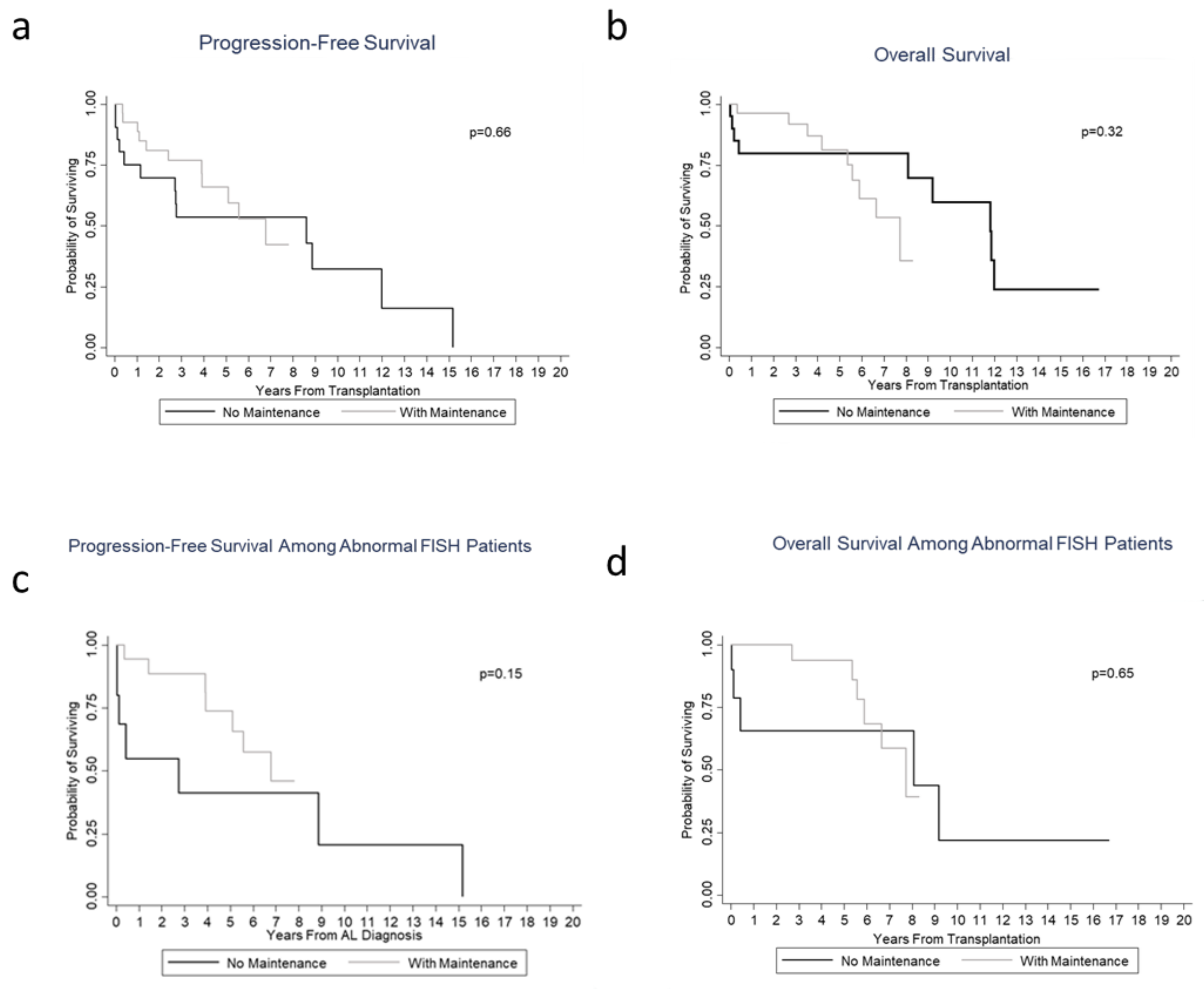
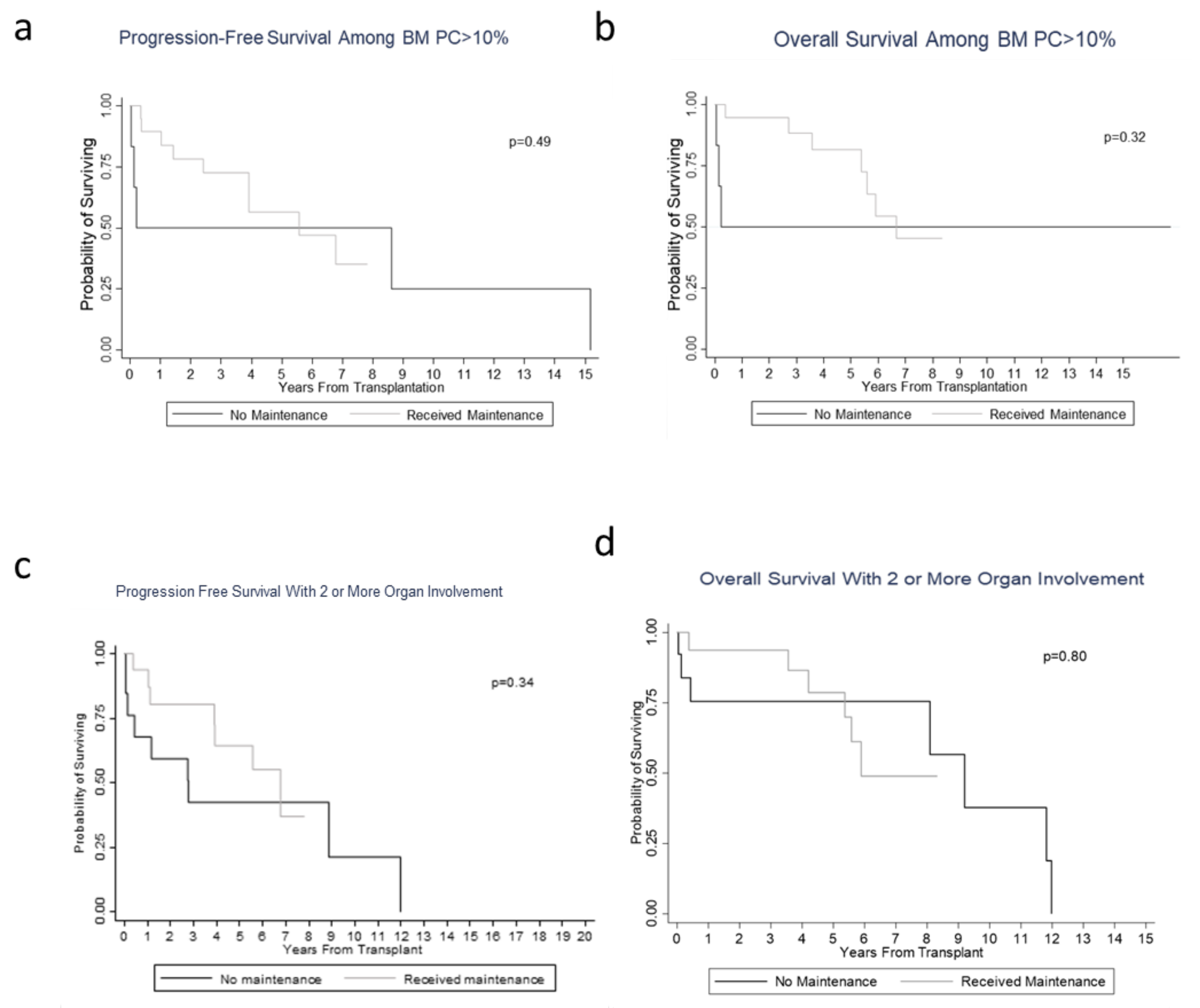
3.2. Hematological/Organ Response and Survival/Prognosis Outcomes
3.3. Toxicity Profiles in the Maintenance Group
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kyle, R.A.; Linos, A.; Beard, C.M.; Linke, R.P.; Gertz, M.A.; O’Fallon, W.M.; Kurland, L.T. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992, 79, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2014, 89, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Systemic Light Chain Amyloidosis. Version 1. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/amyloidosis.pdf. (accessed on 1 February 2019).
- Merlini, G.; Seldin, D.C.; Gertz, M.A. Amyloidosis: Pathogenesis and New Therapeutic Options. J. Clin. Oncol. 2011, 29, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Desport, E.; Bridoux, F.; Sirac, C.; Delbes, S.; Bender, S.; Fernandez, B.; Quellard, N.; Lacombe, C.; Goujon, J.-M.; Lavergne, D.; et al. AL Amyloidosis. Orphanet J. Rare Dis. 2012, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Comenzo, R.L.; Vosburgh, E.; Falk, R.H.; Sanchorawala, V.; Reisinger, J.; Dubrey, S.; O’Hara, C. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: Survival and responses in 25 patients. Blood 1998, 91, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Wei, L.; Bustamante, L.; Elder, P.; Falk, W. Improved Treatment Related Mortality in Patients with Primary Systemic Amyloidosis (AL Amyloidosis) Undergoing Autologous Hematopoietic Stem Cell Transplant (aHSCT). Arch. Hematol. Blood Dis. 2019, 2, 12–18. [Google Scholar]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Warsame, R.; Kumar, S.K.; Gertz, M.A.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Leung, N.; Dingli, D.; Lust, J.A.; Ketterling, R.P.; et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015, 5, e310. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Comenzo, R.L. High-dose melphalan and stem cell transplantation in systemic AL amyloidosis in the era of novel anti-plasma cell therapy: A comprehensive review. Bone Marrow Transplant. 2018, 54, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Gertz, M.A.; Kyle, R.A.; Lacy, M.Q.; Burritt, M.F.; Therneau, T.M.; Greipp, P.R.; Witzig, T.E.; Lust, J.A.; Rajkumar, S.V.; et al. Serum Cardiac Troponins and N-Terminal Pro-Brain Natriuretic Peptide: A Staging System for Primary Systemic Amyloidosis. J. Clin. Oncol. 2004, 22, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised Prognostic Staging System for Light Chain Amyloidosis Incorporating Cardiac Biomarkers and Serum Free Light Chain Measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Landau, H.J.; Hassoun, H.T.; Rosenzweig, M.A.; Maurer, M.; Liu, J.; Flombaum, C.; Di Bello, C.; Hoover, E.L.; Riedel, E.; Giralt, S.; et al. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia 2013, 27, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Landau, H.; Lahoud, O.; Devlin, S.; Lendvai, N.; Chung, D.J.; Dogan, A.; Landgren, C.O.; Giralt, S.; Hassoun, H. Pilot Study of Bortezomib and Dexamethasone Pre- and Post-Risk-Adapted Autologous Stem Cell Transplantation in AL Amyloidosis. Biol. Blood Marrow Transplant. 2020, 26, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.P.; Schrier, S.L.; Lafayette, R.A.; Arai, S.; Witteles, R.; Liedtke, M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 2017, 130, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Merlini, G.; Palladini, G. Novel Therapies in Light Chain Amyloidosis. Kidney Int. Rep. 2018, 3, 530–541. [Google Scholar] [CrossRef]
- Palladini, G.; Kastritis, E.; Maurer, M.S.; Zonder, J.A.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Bumma, N.; Kaufman, J.L.; et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: Safety run-in results of ANDROMEDA. Blood 2020, 136, 71–80. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 50) | No Maintenance Therapy (n = 22) | Maintenance Therapy (n = 28) | p-Value |
|---|---|---|---|---|
| Median age at diagnosis (range), years | 58 (33–71) | 58 (33–71) | 58 (39–70) | 0.990 |
| Gender, male, n (%) | 33 (66.0) | 15 (68.2) | 18 (64.3) | 0.773 |
| Specific induction regimen received, n (%) | Lenalidomide–dexamethasone 5 (10%) Melphalan–prednisone 4 (8%) Bortezomib–dexamethasone 15 (30%) Bortezomib–lenalidomide–dexamethasone 2 (4%) Cyclophosphamide–bortezomib–dexamethasone 14 (28%) None 10 (20%) | |||
| Specific maintenance therapy used, n (%) Immunomodulator (lenalidomide, thalidomide), bortezomib | 0 (0) 0 (0) | 24 (86) 4(14) | ||
| Melphalan dose received, 140 mg/m2 200 mg/m2 | 23 (46.0) 27 (54.0) | 11 (50.0) 11 (50.0) | 12 (42.9) 16 (57.1) | 0.620 |
| Light chain restriction (kappa) | 14 (28) | 4 (18.2) | 10 (35.7) | 0.215 |
| Light chain restriction (lambda) | 35 (70) | 18 (81.8) | 17 (60.7) | 0.131 |
| dFLC mg/dla median (range) | 17.9 (1209–7001) | 15.9 (300–607) | 25 (1209–7001) | 0.640 |
| BMPCb <10% ≥10% | 24 (48%) 26 (52%) | 16 (72.7) 6 (27.3) | 8 (28.6) 20 (71.4) | 0.004 |
| Urine total protein, mg/24 h, median (range) | 6849 (0–81,921) | 10,308 (0–81,921) | 3758 (0–22,500) | 0.06 |
| No. of involved organs, median (range) | 2 (0–5) | 1 (0–4) | 2 (1–5) | 0.910 |
| Cardiac involvement, n (%) | 18 (36.0) | 10 (45.5) | 8 (28.6) | 0.217 |
| Renal involvement, n (%) | 40 (80.0) | 19 (86.4) | 21 (75.0) | 0.319 |
| NT-proBNP ≥ 332 ng/L, n (%) | 19 (70.4) | 8 (72.7) | 11 (68.8) | 0.824 |
| NT-proBNP ≥ 1800 ng/L, n (%) | 11 (40.7) | 6 (54.5) | 5 (31.3) | 0.226 |
| Mayo stage (2012), n (%) | 0.460 | |||
| I | 13 (50.0) | 5 (50.0) | 8 (50.0) | |
| II | 7 (26.9) | 4 (40.0) | 3 (18.8) | |
| III | 2 (7.7) | 0 (0.0) | 2 (12.5) | |
| IV | 4 (15.4) | 1 (10.0) | 3 (18.8) | |
| Missing | 24 | 12 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozga, M.; Zhao, Q.; Benson, D.; Elder, P.; Williams, N.; Bumma, N.; Rosko, A.; Chaudhry, M.; Khan, A.; Devarakonda, S.; et al. AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes. J. Clin. Med. 2020, 9, 3778. https://doi.org/10.3390/jcm9113778
Ozga M, Zhao Q, Benson D, Elder P, Williams N, Bumma N, Rosko A, Chaudhry M, Khan A, Devarakonda S, et al. AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes. Journal of Clinical Medicine. 2020; 9(11):3778. https://doi.org/10.3390/jcm9113778
Chicago/Turabian StyleOzga, Michael, Qiuhong Zhao, Don Benson, Patrick Elder, Nita Williams, Naresh Bumma, Ashley Rosko, Maria Chaudhry, Abdullah Khan, Srinivas Devarakonda, and et al. 2020. "AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes" Journal of Clinical Medicine 9, no. 11: 3778. https://doi.org/10.3390/jcm9113778
APA StyleOzga, M., Zhao, Q., Benson, D., Elder, P., Williams, N., Bumma, N., Rosko, A., Chaudhry, M., Khan, A., Devarakonda, S., Kahwash, R., Vallakati, A., Campbell, C., Parikh, S. V., Almaani, S., Prosek, J., Bittengle, J., Pfund, K., LoRusso, S., ... Sharma, N. (2020). AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes. Journal of Clinical Medicine, 9(11), 3778. https://doi.org/10.3390/jcm9113778







