Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Binary Regression Models
3.2. Logistic Regression Models
3.3. Risk Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Benitez, G.; Desai, J.R.; Xu, S.; Goodrich, G.K.; Schroeder, E.B.; Nichols, G.A.; Segal, J.; Butler, M.G.; Karter, A.J.; Steiner, J.F.; et al. Preventable Major Cardiovascular Events Associated with Uncontrolled Glucose, Blood Pressure, and Lipids and Active Smoking in Adults with Diabetes with and without Cardiovascular Disease: A Contemporary Analysis. Diabetes Care 2015, 38, 905–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmala, W.; Colonna, P.; Przewlocka-Kosmala, M.; Mazurek, W. Right ventricular dysfunction in asymptomatic diabetic patients. Diabetes Care 2004, 27, 2736–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, M. Diagnosis and Management of Left Ventricular Diastolic Dysfunction in the Hypertensive Patient. Am. J. Hypertens. 2011, 24, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Tajik, A. Evaluation of Diastolic Filling of Left Ventricle in Health and Disease: Doppler Echocardiography Is the Clinician’s Rosetta Stone. J. Am. Coll. Cardiol. 1997, 30, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.S.; Smiseth, O.A.; Dokainish, H.; Abudiab, M.M.; Schutt, R.C.; Kumar, A.; Sato, K.; Harb, S.; Gude, E.; Remme, E.W.; et al. Estimating Left Ventricular Filling Pressure by Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1937–1948. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Blonde, L. Benefits and Risks for Intensive Glycemic Control in Patients with Diabetes Mellitus. Am. J. Med. Sci. 2012, 343, 17–20. [Google Scholar] [CrossRef]
- Turnbull, F.M.; Abraira, C.; Anderson, R.J.; Byington, R.P.; Chalmers, J.P.; Duckworth, W.C.; Evans, G.W.; Gerstein, H.C.; Holman, R.R.; Moritz, T.E.; et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetology 2009, 52, 2288–2298. [Google Scholar] [CrossRef]
- Mattila, T.K.; De Boer, A. Influence of Intensive versus Conventional Glucose Control on Microvascular and Macrovascular Complications in Type 1 and 2 Diabetes Mellitus. Drugs 2010, 70, 2229–2245. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.K.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacokinetic and Pharmacodynamic Profile of Empagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor. Clin. Pharmacokinet. 2014, 53, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef] [Green Version]
- Braha, A.; Timar, B.; Diaconu, L.; Lupusoru, R.; Vasiluta, L.; Sima, A.; Vlad, A.; Munteanu, M.; Albai, A.; Cipu, D.; et al. Dynamics of Epicardiac Fat and Heart Function in Type 2 Diabetic Patients Initiated with SGLT-2 Inhibitors. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Faden, G.; Faganello, G.; De Feo, S.; Berlinghieri, N.; Tarantini, L.; Di Lenarda, A.; Faggiano, P.; Cioffi, G. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: Data from the SHORTWAVE study. Diabetes Res. Clin. Pr. 2013, 101, 309–316. [Google Scholar] [CrossRef]
- Celentano, A.; Vaccaro, O.; Tammaro, P.; Galderisi, M.; Crivaro, M.; Oliviero, M.; Imperatore, G.; Palmieri, V.; Iovino, V.; Riccardi, G.; et al. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am. J. Cardiol. 1995, 76, 1173–1176. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Exiara, T.; Konstantis, A.; Papazoglou, L.; Kouroupi, M.; Kalpaka, A.; Mporgi, L.; Risggits, A.; Filippidou, E.; Terzi, S.; Papanastasiou, S. LEFT VENTRICULAR DIASTOLIC DYSFUNCTION IN DIABETES MELLITUS TYPE 2: PP.17.147. J. Hypertens. 2010, 28, e294. [Google Scholar] [CrossRef]
- Patil, V.C.; Shah, K.B.; Vasani, J.D.; Shetty, P.; Patil, H.V. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J. Cardiovasc. Dis. Res. 2011, 2, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, N.R.; Sharma, S.; Karki, P.; Shrestha, N.K.; Acharya, P. Echocardiographic evaluation of diastolic function in asymptomatic type 2 diabetes. J. Nepal Med. Assoc. 2009, 48, 20–23. [Google Scholar] [CrossRef]
- Ernande, L.; Bergerot, C.; Rietzschel, E.-R.; De Buyzere, M.L.; Thibault, H.; PignonBlanc, P.G.; Croisille, P.; Ovize, M.; Groisne, L.; Moulin, P.; et al. Diastolic Dysfunction in Patients with Type 2 Diabetes Mellitus: Is It Really the First Marker of Diabetic Cardiomyopathy? J. Am. Soc. Echocardiogr. 2011, 24, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.V.; Chilton, R.J. EMPA-REG Outcome: The Cardiologist’s Point of View. Am. J. Cardiol. 2017, 120, S53–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekeran, H.; Lytvyn, Y.; Cherney, D.Z. Sodium–glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: The emerging role of natriuresis. Kidney Int. 2016, 89, 524–526. [Google Scholar] [CrossRef] [Green Version]
- Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 73. [Google Scholar] [CrossRef]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Goodman, S.G. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Wang, D.; Luo, Y.; Wang, X.; Orlicky, D.J.; Myakala, K.; Yang, P.-Y.; Levi, M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. Int. J. Mol. Sci. 2018, 19, 137. [Google Scholar] [CrossRef] [Green Version]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients with Preserved Ejection Fraction. Circ. Hear. Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, R.; Daimon, M.; Miyazaki, T.; Kawata, T.; Miyazaki, S.; Maruyama, M.; Chiang, S.-J.; Suzuki, H.; Ito, C.; Sato, F.; et al. Influencing factors on cardiac structure and function beyond glycemic control in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Alsaied, T.; Moore, R.A.; Lang, S.M.; Truong, V.; Lubert, A.M.; Veldtman, G.R.; Averin, K.; Dillman, J.R.; Trout, A.T.; Mazur, W.; et al. Myocardial fibrosis, diastolic dysfunction and elevated liver stiffness in the Fontan circulation. Open Hear. 2020, 7, e001434. [Google Scholar] [CrossRef]
- Wu, F.M.; Opotowsky, A.R.; Raza, R.; Harney, S.; Ukomadu, C.; Landzberg, M.J.; Valente, A.M.; Breitbart, R.E.; Singh, M.N.; Gauvreau, K.; et al. Transient Elastography May Identify Fontan Patients with Unfavorable Hemodynamics and Advanced Hepatic Fibrosis. Congenit. Hear. Dis. 2014, 9, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Liangpunsakul, S. Cirrhotic cardiomyopathy: Review of pathophysiology and treatment. Hepatol. Int. 2014, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 69, 463–473. [Google Scholar] [CrossRef] [PubMed]


| Variable | Baseline | At Six-Month Follow-Up | p |
|---|---|---|---|
| Glycemia (mg/dL) b | 213.1 ± 69.5 | 152.8 ± 35 | <0.001 * |
| Total cholesterol (mg/dL) b | 197.1 ± 61.2 | 191.1 ± 46.5 | 0.07 |
| Triglycerides (mg/dL) a | 170 (5; 887) | 150 (58; 1397) | 0.4 |
| LDLc (mg/dL) b | 120.7 ± 47.1 | 107.4 ± 39.4 | 0.3 |
| HDLc (mg/dL) a | 39 (17; 89) | 42 (19; 90) | 0.03 * |
| women b | 39.8 ± 11.8 | 45.1 ± 12.4 | 0.1 |
| men a | 39 (17; 89) | 41.5 (19; 90) | 0.03 * |
| Uric acid (mg/dL) b | 4.9 ± 1.2 | 4.6 ± 1.1 | 0.1 |
| eGFR (mL/min/1.73 m2) b | 86.1 ± 14.4 | 94.2 ± 13.2 | 0.007 * |
| HbA1c (%) b | 8.6 ± 1.1 | 7.9 ± 1.2 | 0.003 * |
| UACr (mg/g) a | 15.4 (5.5; 471.1) | 16.7 (1.3; 928.3) | 0.9 |
| Weight (kg) b | 99.4 ± 4.7 | 94.7 ± 14.9 | 0.0001 * |
| women b | 94.5 ± 15.3 | 90.1 ± 15.7 | <0.0001 * |
| men b | 103.6 ± 16.3 | 98.7 ± 13.3 | <0.0001 * |
| BMI (kg/m2) b | 34.9 ± 4.7 | 33.3 ± 4.6 | <0.0001 * |
| women b | 36.6 ± 5 | 35 ± 5.3 | 0.0002 * |
| men b | 33.4 ± 4 | 31.9 ± 3.5 | <0.0001 * |
| waist (cm) b | 116 ± 11.8 | 114.3 ± 11.5 | 0.03 * |
| women b | 115.6 ± 10.1 | 115 ± 11 | 0.5 |
| men b | 116.2 ± 13.2 | 113.7 ± 12.1 | 0.02 * |
| AST (u/L) | 27 (12; 80) | 21 (9; 45) | 0.01 * |
| ALT (u/L) | 47 (20; 174) | 34.5 (21; 74) | 0.002 * |
| Systolic BP (mmHg) | 142.3 ± 21.5 | 137.8 ± 17 | 0.2 |
| Diastolic BP (mmHg) | 85 (65; 125) | 85 (70; 105) | 0.9 |
| The epicardial fat thickness on echo cardiac (mm) b | 6 ± 1.3 | 4.2 ± 1.4 | 0.0001 * |
| women b | 6 ± 1.3 | 4 ± 1 | <0.0001 * |
| men b | 6 ± 1.4 | 4.3 ± 1.7 | 0.0002 * |
| EPI volume (cm3) b | 37.6 ± 16.3 | 20.6 ± 7.3 | <0.0001 * |
| women b | 35.8 ± 17 | 19.6 ± 5 | 0.0003 * |
| men b | 9.5 ± 16.1 | 21.4 ± 8.8 | <0.0001 * |
| L4 visceral fat thickness (mm) b | 64 ± 15.8 | 65.4 ± 14.4 | 0.6 |
| women b | 69.1 ± 14.5 | 68.7 ± 13.9 | 0.8 |
| men b | 61.1 ± 17.1 | 62.8 ± 14.5 | 0.3 |
| L4 fat volume (cm3) b | 37.8 ± 29.8 | 41.9 ± 18.5 | 0.4 |
| women b | 35.5 ± 27.6 | 45.9 ± 17.4 | 0.07 |
| men b | 42.9 ± 31.5 | 38.2 ± 19.1 | 0.5 |
| Mediastinal fat thickness (mm) b | 24.4 ± 6.8 | 27.8 ± 6.9 | 0.02 * |
| women b | 25 ± 5.1 | 26.5 ± 6.5 | 0.1 |
| men b | 25.8 ± 7.6 | 28.9 ± 7.1 | 0.1 |
| CAP (dB/m) a | 368 (240; 400) | 310 (190; 400) | 0.0003 * |
| women b | 362.6 ± 31.7 | 316.1 ± 52 | 0.001 * |
| men a | 373 (240; 400) | 310 (190; 400) | 0.03 * |
| Fibroscan (liver stiffness) (kPa) a | 7.2 (3.5; 43.8) | 6.7 (3.3; 15.3) | 0.09 |
| women a | 7.4 (3,5; 43.8) | 7.3 (3.4; 15.3) | 0.3 |
| men a | 6.8 (3.9; 20) | 6.2 (3.3; 12.1) | 0.1 |
| Variable | Exp (β) | p |
|---|---|---|
| ΔHbA1c (%) ** | 1.85 | 0.03 * |
| ΔBMI (kg/m2) ** | 0.61 | 0.06 |
| ΔGFR (mL/min/1.73 m2) ** | 0.91 | 0.02 * |
| Age (years) ** | 0.96 | 0.3 |
| Diabetes duration (years) ** | 0.81 | 0.01 * |
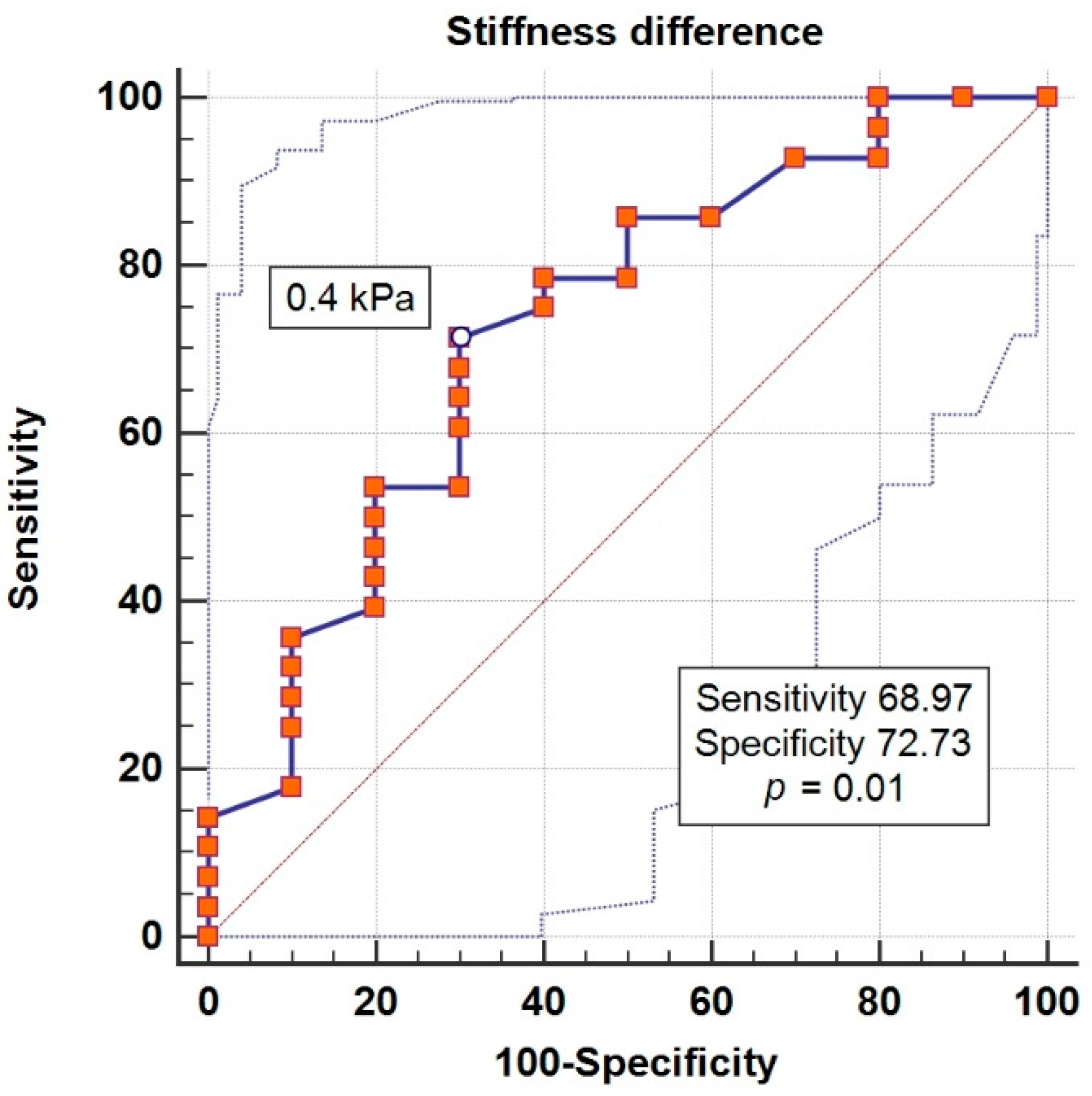
| Δ liver stiffness (kPa) *** | 1.24 | 0.04 * |
| ΔCAP (dB/m) *** | 0.99 | 0.4 |
| Subcutaneous Adipose Tissue Predominance | Visceral Adipose Tissue Predominance | |||
|---|---|---|---|---|
| At Six Months | At One Year | At Six Months | At One Year | |
| Relative risk | 0.41 | 1.25 | 2.4 | 0.79 |
| CI 95% | 0.15–1.08 | 0.77–2.03 | 0.9–6.3 | 0.49–1.28 |
| statistic z | 1.791 | 0.932 | 1.7 | 0.93 |
| p | 0.07 | 0.35 | 0.07 | 0.3 |
| Odds ratio | 0.27 | 2.28 | 3.61 | 0.43 |
| CI 95% | 0.06–1.21 | 0.5–10.44 | 0.8–15.8 | 0.09–1.99 |
| statistic z | 1.698 | 0.932 | 1.6 | 1.066 |
| p | 0.08 | 0.28 | 0.08 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braha, A.; Albai, A.; Timar, R.; Diaconu, L.; Vasiluță, L.; Cipu, D.; Timar, B.; Sima, A. Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes. J. Clin. Med. 2020, 9, 3779. https://doi.org/10.3390/jcm9113779
Braha A, Albai A, Timar R, Diaconu L, Vasiluță L, Cipu D, Timar B, Sima A. Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes. Journal of Clinical Medicine. 2020; 9(11):3779. https://doi.org/10.3390/jcm9113779
Chicago/Turabian StyleBraha, Adina, Alin Albai, Romulus Timar, Laura Diaconu, Lucian Vasiluță, Daniela Cipu, Bogdan Timar, and Alexandra Sima. 2020. "Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes" Journal of Clinical Medicine 9, no. 11: 3779. https://doi.org/10.3390/jcm9113779
APA StyleBraha, A., Albai, A., Timar, R., Diaconu, L., Vasiluță, L., Cipu, D., Timar, B., & Sima, A. (2020). Factors Associated with the Remission of Type 1 Diastolic Dysfunction after Dapagliflozin Treatment in Patients with Type 2 Diabetes. Journal of Clinical Medicine, 9(11), 3779. https://doi.org/10.3390/jcm9113779





