High Molecular Weight Hyaluronan Promotes Corneal Nerve Growth in Severe Dry Eyes
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Participants
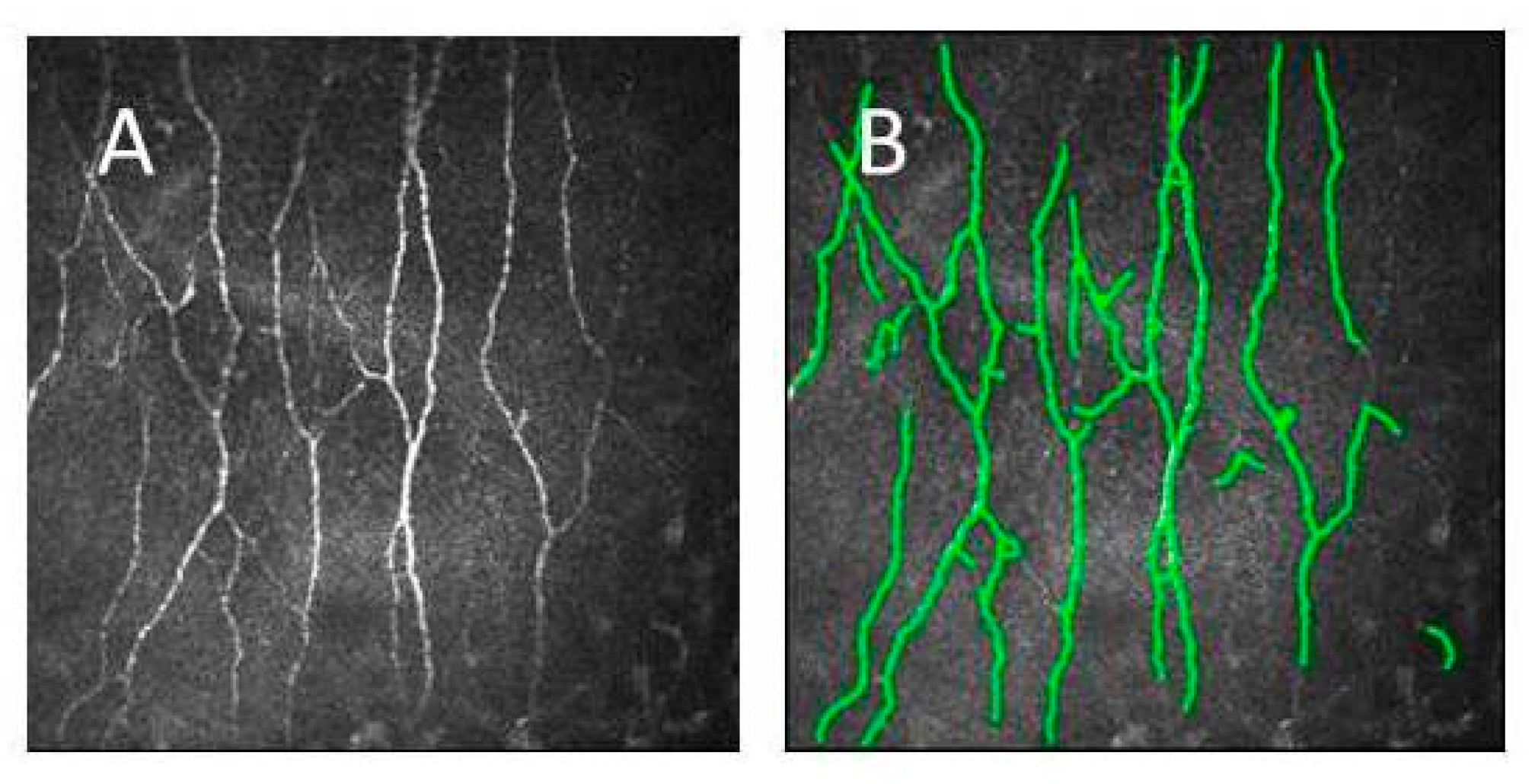
2.3. Confocal Scanning Laser Microscopy
2.4. Statistical Analysis
3. Results
3.1. Participant Demography
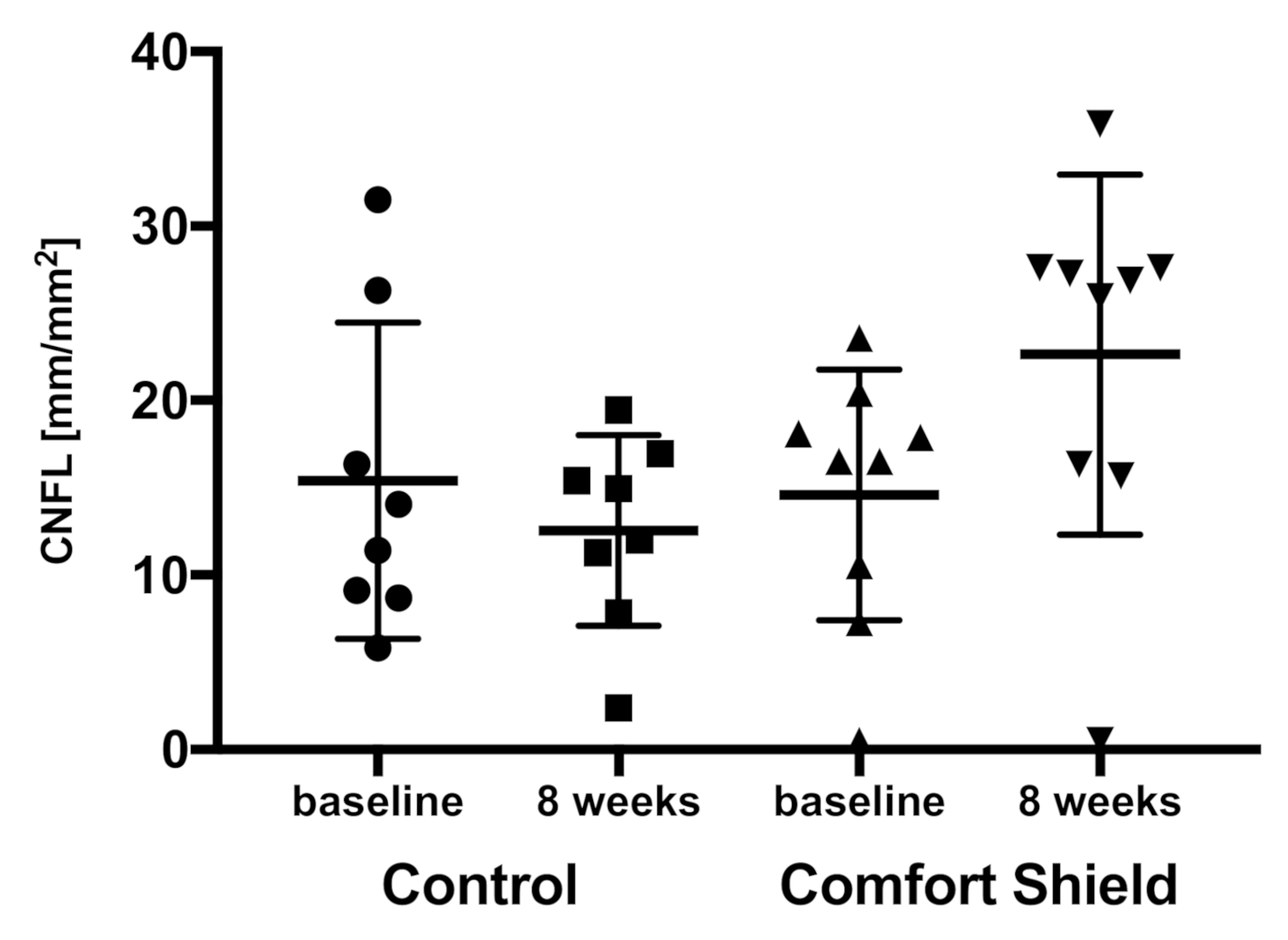
3.2. Confocal Microscopy Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lierheim, W.G.K. Why Chain Length of Hyaluronan in Eye Drops Matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; van Setten, G.-B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- Baudouin, C. A new approach for better comprehension of diseases of the ocular surface. J. Fr. Ophtalmol. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Baudouin, C.; Irkec, M.; Messmer, E.M.; Benitez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Belmonte, C. Pain, Dryness, and Itch Sensations in Eye Surface Disorders Are Defined By a Balance Between Inflammation and Sensory Nerve Injury. Cornea 2019, 38 (Suppl. 1), S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S. The neurotrophic factor concept: A reexamination. J. Neurosci. 1993, 13, 2739–2748. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [Green Version]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; von Andrian, U.H. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015, 36, 578–604. [Google Scholar] [CrossRef] [Green Version]
- Dastjerdi, M.H.; Dana, R. Corneal nerve alterations in dry eye-associated ocular surface disease. Int. Ophthalmol. Clin. 2009, 49, 11–20. [Google Scholar] [CrossRef]
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872. [Google Scholar] [CrossRef]
- Purves, D. The trophic theory of neural concentrations. Trends Neurosci. 1986, 9, 486–489. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchetti, M.; Lambiase, A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res. 2017, 12, 1220–1224. [Google Scholar] [PubMed]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef] [PubMed]
- van Setten, G.-B. Impact of Attrition, Intercellular Shear in Dry Eye Disease: When Cells are Challenged and Neurons are Triggered. Int. J. Mol. Sci. 2020, 21, 4333. [Google Scholar] [CrossRef]
- del Castillo, J.M.B.; Wasfy, M.A.S.; Fernandez, C.; Garcia-Sanchez, J. An In Vivo Confocal Masked Study on Corneal Epithelium and Subbasal Nerves in Patients with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3030–3035. [Google Scholar] [CrossRef] [Green Version]
- Villani, E.; Galimberti, D.; Viola, F.; Mapelli, C.; Ratiglia, R. The cornea in Sjogren’s syndrome: An in vivo confocal study. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2017–2022. [Google Scholar] [CrossRef]
- Labbe, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef]
- Tepelus, T.C.; Chiu, G.B.; Huang, J.; Huang, P.; Sadda, S.R.; Irvine, J.; Lee, O.L. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: A preliminary study. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2017, 255, 1771–1778. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ibrahim, O.M.A.; Kojima, T.; Dogru, M.; Shimazaki, J.; Tsubota, K. Corneal In Vivo Laser-Scanning Confocal Microscopy Findings in Dry Eye Patients with Sjogren’s Syndrome. Diagnostics (Basel) 2020, 10, 497. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Groener, J.B.; Kender, Z.; Fleming, T.; Bischoff, S.; Jende, J.; Schumann, C.; Ries, S.; Bendszus, M.; Schuh-Hofer, S.; et al. Deep phenotyping neuropathy: An underestimated complication in patients with pre-diabetes and type 2 diabetes associated with albuminuria. Diabetes Res. Clin. Pract. 2018, 146, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Massaro-Giordano, G.; Nubile, M.; Sacchetti, M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. J. Cell. Physiol. 2017, 232, 717–724. [Google Scholar] [CrossRef]
- Galor, A.; Batawi, H.; Felix, E.R.; Margolis, T.P.; Sarantopoulos, K.D.; Martin, E.R.; Levitt, R.C. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br. J. Ophthalmol. 2016, 100, 745–749. [Google Scholar] [CrossRef]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef]
- Gomis, A.; Pawlak, M.; Balazs, E.A.; Schmidt, R.F.; Belmonte, C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004, 50, 314–326. [Google Scholar] [CrossRef]
- Caires, R.; Luis, E.; Taberner, F.J.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Balazs, E.A.; Gomis, A.; Belmonte, C.; de la Pena, E. Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain. Nat. Commun. 2015, 6, 8095. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, L.F.; Khomula, E.V.; Araldi, D.; Levine, J.D. CD44 Signaling Mediates High Molecular Weight Hyaluronan-Induced Antihyperalgesia. J. Neurosci. 2018, 38, 308–321. [Google Scholar] [CrossRef]
- Casini, P.; Nardi, I.; Ori, M. RHAMM mRNA expression in proliferating and migrating cells of the developing central nervous system. Gene Expr. Patterns 2010, 10, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.; Sherman, L.S. Neural stem cell niches: Roles for the hyaluronan-based extracellular matrix. Front. Biosci. 2011, 3, 1165–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthoff, R.F.; Zhivov, A.; Stachs, O. In vivo confocal microscopy, an inner vision of the cornea—A major review. Clin. Exp. Ophthalmol. 2009, 37, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Dohlman, T.H.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology 2015, 122, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [Green Version]
- Kowtharapu, B.S.; Stachs, O. Corneal Cells: Fine-tuning Nerve Regeneration. Curr. Eye Res. 2020, 45, 291–302. [Google Scholar] [CrossRef]
- van Setten, G.B.; Baudouin, C.; Horwath-Winter, J.; Bohringer, D.; Stachs, O.; Toker, E.; Al-Zaaidi, S.; Benitez-Del-Castillo, J.M.; Beck, R.; Al-Sheikh, O.; et al. The HYLAN M Study: Efficacy of 0.15% High Molecular Weight Hyaluronan Fluid in the Treatment of Severe Dry Eye Disease in a Multicenter Randomized Trial. J. Clin. Med. 2020, 9, 3536. [Google Scholar] [CrossRef]
- Korb, D.R.; Herman, J.P.; Blackie, C.A.; Scaffidi, R.C.; Greiner, J.V.; Exford, J.M.; Finnemore, V.M. Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea 2010, 29, 377–383. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kutsuna, M.; Uno, T.; Zheng, X.; Kodama, T.; Ohashi, Y. Marx line: Fluorescein staining line on the inner lid as indicator of meibomian gland function. Am. J. Ophthalmol. 2006, 141, 669–675. [Google Scholar] [CrossRef]
- Karpecki, P.M. Why dry eye trials often fail: From disease variability to confounding underlying conditions, there are countless reasons why new dry eye drugs have come up short in FDA testing. Rev. Optom. 2013, 150, 50–56. [Google Scholar]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkec, M.; Benitez del Castillo, J.; Geerling, G.; Labetoulle, M.; Bonini, S.; ODISSEY European Consensus Group Members. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Zhivov, A.; Allgeier, S.; Winter, K.; Ziegler, I.; Bruggemann, J.; Strom, A.; Peschel, S.; Kohler, B.; et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014, 63, 2454–2463. [Google Scholar] [CrossRef] [Green Version]
- Stachs, O.; Guthoff, R.F.; Aumann, S. In Vivo Confocal Scanning Laser Microscopy. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 263–284. [Google Scholar]
- Winter, K.; Scheibe, P.; Kohler, B.; Allgeier, S.; Guthoff, R.F.; Stachs, O. Local Variability of Parameters for Characterization of the Corneal Subbasal Nerve Plexus. Curr. Eye Res. 2016, 41, 186–198. [Google Scholar] [CrossRef]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Moscardelli, F.; Buzzi, M.; Versura, P.; Campos, E.C. In Vivo Confocal Microscopy Automated Morphometric Analysis of Corneal Subbasal Nerve Plexus in Patients With Dry Eye Treated With Different Sources of Homologous Serum Eye Drops. Cornea 2019, 38, 1412–1417. [Google Scholar] [CrossRef]
- Malik, R.A.; Kallinikos, P.; Abbott, C.A.; van Schie, C.H.; Morgan, P.; Efron, N.; Boulton, A.J. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003, 46, 683–688. [Google Scholar] [CrossRef]
- Mocan, M.C.; Durukan, I.; Irkec, M.; Orhan, M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea 2006, 25, 769–773. [Google Scholar] [CrossRef]
- Hertz, P.; Bril, V.; Orszag, A.; Ahmed, A.; Ng, E.; Nwe, P.; Ngo, M.; Perkins, B.A. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011, 28, 1253–1260. [Google Scholar] [CrossRef]
- Stem, M.S.; Hussain, M.; Lentz, S.I.; Raval, N.; Gardner, T.W.; Pop-Busui, R.; Shtein, R.M. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J. Diabetes Complicat. 2014, 28, 658–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Dehghani, C.; Pritchard, N.; Edwards, K.; Russell, A.W.; Malik, R.A.; Efron, N. Corneal and Retinal Neuronal Degeneration in Early Stages of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6365–6373. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N.S.; Allgeier, S.; Guimaraes, P.; Badian, R.A.; Ruggeri, A.; Kohler, B.; Utheim, T.P.; Peebo, B.; Peterson, M.; Dahlin, L.B.; et al. Reduced Corneal Nerve Fiber Density in Type 2 Diabetes by Wide-Area Mosaic Analysis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6318–6327. [Google Scholar] [CrossRef] [Green Version]
- Kalteniece, A.; Ferdousi, M.; Petropoulos, I.; Azmi, S.; Adam, S.; Fadavi, H.; Marshall, A.; Boulton, A.J.M.; Efron, N.; Faber, C.G.; et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci. Rep. 2018, 8, 3283. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. The structure and function of hyaluronan: An overview. Immunol. Cell Biol. 1996, 74, A1–A7. [Google Scholar] [CrossRef] [PubMed]
- Asari, A.; Miyauchi, S.; Takahashi, T.; Kohno, K.; Uchiyama, Y. Localization of hyaluronic acid, chondroitin sulfate, and CD44 in rabbit cornea. Arch. Histol. Cytol. 1992, 55, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, L.E.; Schwartz, D.M.; Hwang, D.G.; Howes, E.L.; Stern, R. Hyaluronan and CD44 in the human cornea and limbal conjunctiva. Exp. Eye Res. 1998, 67, 481–484. [Google Scholar] [CrossRef]
- Zhu, S.N.; Nolle, B.; Duncker, G. Expression of adhesion molecule CD44 on human corneas. Br. J. Ophthalmol. 1997, 81, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, M.; Schledzewski, K.; Hansen, B.; Goerdt, S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 2003, 120, 361–369. [Google Scholar] [CrossRef]
- Harris, E.N.; Baker, E. Role of the Hyaluronan Receptor, Stabilin-2/HARE, in Health and Disease. Int. J. Mol. Sci. 2020, 21, 3504. [Google Scholar] [CrossRef]
- Hansen, I.M.; Ebbesen, M.F.; Kaspersen, L.; Thomsen, T.; Bienk, K.; Cai, Y.; Malle, B.M.; Howard, K.A. Hyaluronic Acid Molecular Weight-Dependent Modulation of Mucin Nanostructure for Potential Mucosal Therapeutic Applications. Mol. Pharm. 2017, 14, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Delmage, J.M.; Powars, D.R.; Jaynes, P.K.; Allerton, S.E. The selective suppression of immunogenicity by hyaluronic acid. Ann. Clin. Lab. Sci. 1986, 16, 303–310. [Google Scholar] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef] [Green Version]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds A Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol Res. 2014, 58, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Toole, B.P. Hyaluronan and its binding proteins, the hyaladherins. Curr. Opin. Cell Biol. 1990, 2, 839–844. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993, 7, 1233–1241. [Google Scholar] [CrossRef]
- Evanko, S.P.; Tammi, M.I.; Tammi, R.H.; Wight, T.N. Hyaluronan-Dependent Pericellular Matrix. Adv. Drug Deliv. Rev. 2007, 59, 1351–1365. [Google Scholar] [CrossRef] [Green Version]
- Agren, U.M.; Tammi, R.H.; Tammi, M.I. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic. Biol. Med. 1997, 23, 996–1001. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Zhou, Q. Efficacy of Sodium Hyaluronate in Murine Diabetic Ocular Surface Diseases. Cornea 2017, 36, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, F.; Forbice, E.; Romano, V.; Angi, M.; Romano, M.R.; Filippelli, M.E.; Di Iorio, R.; Costagliola, C. Neurotrophic keratitis. Ophthalmologica 2014, 231, 191–197. [Google Scholar] [CrossRef]
- Ambrósio, R., Jr.; Tervo, T.; Wilson, S.E. LASIK-associated dry eye and neurotrophic epitheliopathy: Pathophysiology and strategies for prevention and treatment. J. Refract. Surg. 2008, 24, 396–407. [Google Scholar] [CrossRef]
- Sarkar, J.; Milani, B.; Kim, E.; An, S.; Kwon, J.; Jain, S. Corneal nerve healing after in situ laser nerve transection. PLoS ONE 2019, 14, e0218879. [Google Scholar] [CrossRef] [PubMed]
- Karacorlu, M.A.; Cakiner, T.; Saylan, T. Corneal sensitivity and correlations between decreased sensitivity and anterior segment pathology in ocular leprosy. Br. J. Ophthalmol. 1991, 75, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Niederer, R.L.; Perumal, D.; Sherwin, T.; McGhee, C.N. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2964–2970. [Google Scholar] [CrossRef]
- Peters, M.J.; Bakkers, M.; Merkies, I.S.; Hoeijmakers, J.G.; van Raak, E.P.; Faber, C.G. Incidence and prevalence of small-fiber neuropathy: A survey in the Netherlands. Neurology 2013, 81, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. The effect of age on the corneal subbasal nerve plexus. Cornea 2005, 24, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [PubMed] [Green Version]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soifer, M.; Starr, C.E.; Mousa, H.M.; Savarain, C.; Perez, V.L. Neurotrophic Keratopathy: Current Perspectives. Curr. Ophthalmol. Rep. 2020, 8, 29–35. [Google Scholar] [CrossRef]
- Nishida, T.; Yanai, R. Advances in treatment for neurotrophic keratopathy. Curr. Opin. Ophthalmol. 2009, 20, 276–281. [Google Scholar] [CrossRef]
- Yanai, R.; Nishida, T.; Chikama, T.; Morishige, N.; Yamada, N.; Sonoda, K.H. Potential New Modes of Treatment of Neurotrophic Keratopathy. Cornea 2015, 34 (Suppl. 11), S121–S127. [Google Scholar] [CrossRef]
- Bitirgen, G.; Ozkagnici, A.; Malik, R.A.; Kerimoglu, H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014, 31, 431–438. [Google Scholar] [CrossRef]
- Barsegian, A.; Lee, J.; Salifu, M.O.; McFarlane, S.I. Corneal Neuropathy: An Underrated Manifestation of Diabetes Mellitus. J. Clin. Endocrinol. Diabetes 2018, 2. [Google Scholar] [CrossRef]
- Zhivov, A.; Winter, K.; Hovakimyan, M.; Peschel, S.; Harder, V.; Schober, H.C.; Kundt, G.; Baltrusch, S.; Guthoff, R.F.; Stachs, O. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS ONE 2013, 8, e52157. [Google Scholar] [CrossRef]
- Schultz, R.O.; Van Horn, D.L.; Peters, M.A.; Klewin, K.M.; Schutten, W.H. Diabetic keratopathy. Trans. Am. Ophthalmol. Soc. 1981, 79, 180–199. [Google Scholar]
- Manaviat, M.R.; Rashidi, M.; Afkhami-Ardekani, M.; Shoja, M.R. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Burda, N.; Mema, V.; Md, E.M.; Selimi, B.; Zhugli, S.; Lenajni, B.; Bunjaku, I. Prevalence of dry eye syndrome at patients with diabetus melitus tip 2, one year retrospective study May 2011–June 2012. J. Acute Dis. 2012, 1, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Bikbova, G.; Oshitari, T.; Baba, T.; Bikbov, M.; Yamamoto, S. Diabetic corneal neuropathy: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 981–987. [Google Scholar] [PubMed] [Green Version]
- Achtsidis, V.; Eleftheriadou, I.; Kozanidou, E.; Voumvourakis, K.I.; Stamboulis, E.; Theodosiadis, P.G.; Tentolouris, N. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care 2014, 37, e210–e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, K.C.; Lam, K.S.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr. Diabetes 2017, 7, e251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, S.L.; Braatvedt, G.D.; Patel, D.V. Impact of diabetes mellitus on the ocular surface: A review. Clin. Exp. Ophthalmol. 2016, 44, 278–288. [Google Scholar] [CrossRef]
- Tavakoli, M.; Kallinikos, P.; Iqbal, A.; Herbert, A.; Fadavi, H.; Efron, N.; Boulton, A.J.M.; Malik, R.A. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011, 28, 1261–1267. [Google Scholar] [CrossRef]
- Azmi, S.; Jeziorska, M.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Marshall, A.; Alam, U.; Asghar, O.; Atkinson, A.; Jones, W.; et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019, 62, 1478–1487. [Google Scholar] [CrossRef] [Green Version]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol 2017, 5, 423–430. [Google Scholar] [CrossRef]

| Test | Baseline | Week 4 | Week 8 |
|---|---|---|---|
| OSDI | X | X | X |
| Dropping frequency | X | X | X |
| BCVA | X | X | X |
| CFS | X | X | X |
| TBUT | X | X | X |
| Schirmer 1 | X | X | |
| Tear osmolarity | X | X | |
| IOP | X | X | |
| LWE, Korb score [48] | (X) | (X) | |
| Yamaguchi score [49] | (X) | (X) | |
| Confocal microscopy | (X) | (X) |
| Comfort Shield n = 8 | Control n = 8 | ||
|---|---|---|---|
| Age (years) | n | 8 | 8 |
| mean (SD) | 59.5 (9.2) | 61.6 (18.4) | |
| min, max | 36, 77 | 47, 73 | |
| Sex n (%) | n | 8 | 8 |
| female | 6 (75) | 6 (75) | |
| male | 2 (25) | 2 (25) | |
| Medical History | n | 8 | 8 |
| Sjögren syndrome | 2 | 3 | |
| rheumatoid disease | 3 | 2 | |
| rheumatoid + thyroid disease | 1 | ||
| thyroid disease | 1 | ||
| Graves disease + betablocker | 1 | ||
| diabetes mellitus + betablocker | 1 | ||
| no dry eye related disease | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Setten, G.-B.; Stachs, O.; Dupas, B.; Turhan, S.A.; Seitz, B.; Reitsamer, H.; Winter, K.; Horwath-Winter, J.; Guthoff, R.F.; Müller-Lierheim, W.G.K. High Molecular Weight Hyaluronan Promotes Corneal Nerve Growth in Severe Dry Eyes. J. Clin. Med. 2020, 9, 3799. https://doi.org/10.3390/jcm9123799
van Setten G-B, Stachs O, Dupas B, Turhan SA, Seitz B, Reitsamer H, Winter K, Horwath-Winter J, Guthoff RF, Müller-Lierheim WGK. High Molecular Weight Hyaluronan Promotes Corneal Nerve Growth in Severe Dry Eyes. Journal of Clinical Medicine. 2020; 9(12):3799. https://doi.org/10.3390/jcm9123799
Chicago/Turabian Stylevan Setten, Gysbert-Botho, Oliver Stachs, Bénédicte Dupas, Semra Akkaya Turhan, Berthold Seitz, Herbert Reitsamer, Karsten Winter, Jutta Horwath-Winter, Rudolf F. Guthoff, and Wolfgang G. K. Müller-Lierheim. 2020. "High Molecular Weight Hyaluronan Promotes Corneal Nerve Growth in Severe Dry Eyes" Journal of Clinical Medicine 9, no. 12: 3799. https://doi.org/10.3390/jcm9123799







