Immortalization of Salivary Gland Epithelial Cells of Xerostomic Patients: Establishment and Characterization of Novel Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Salivary Gland Cell Lines (SGCLs) and HeLa Cells
2.2. LSG Biopsies and Culture of Salivary Gland Epithelial Cells (SGECs)
2.3. Transduction of SGECs by Lentiviral SV40Lt Particles
2.4. RNA Extraction and cDNA Synthesis
2.5. Matrigel Induced 3D Spheroid Cultures
2.6. Real-Time Quantitative and Semi-Quantitative Polymerase Chain Reaction
2.7. Expression of ZO-1, AQP5, and α-Amylase by Western Blot
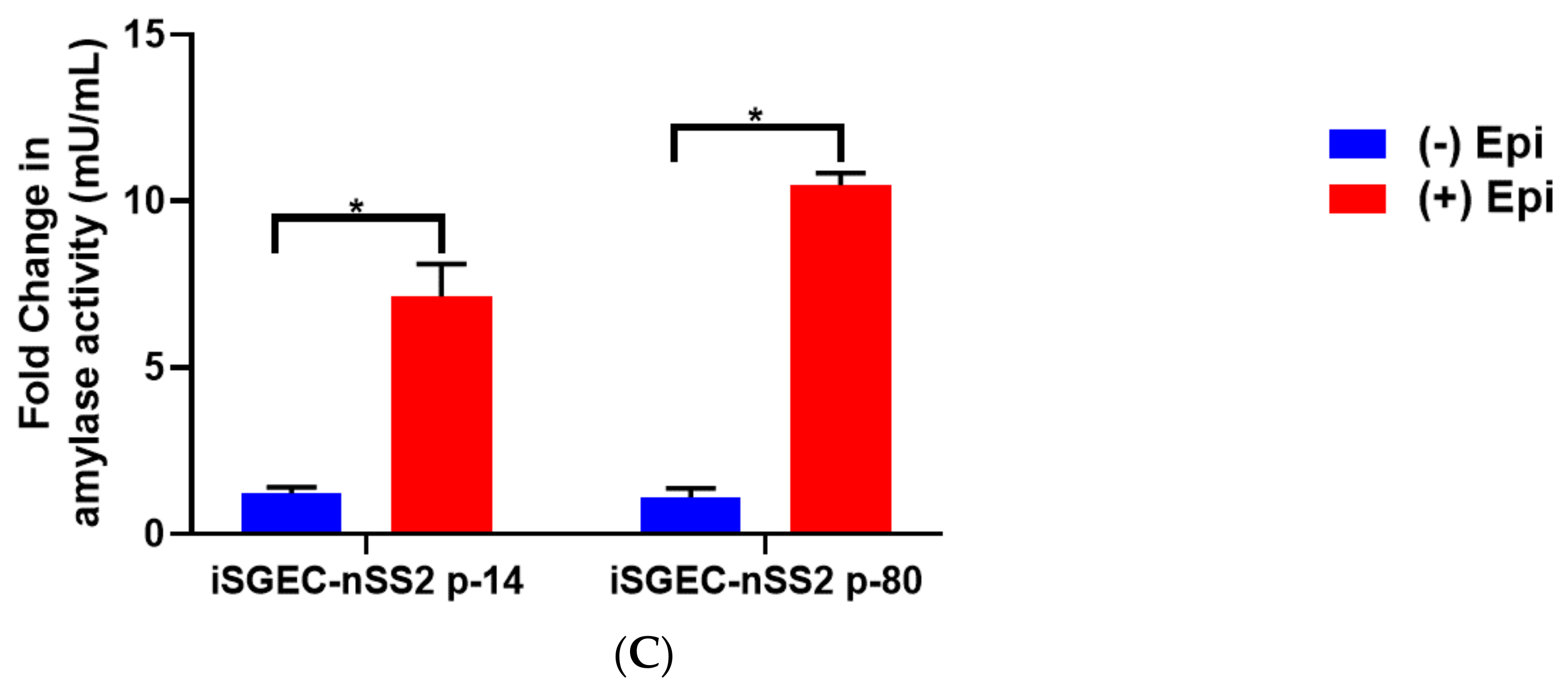
2.8. β-. Adrenergic Stimulation and Measurement of α-Amylase Activity in Supernatant
2.9. Immunocytochemistry (ICC)
3. Data Analysis and Statistics
4. Results
4.1. Primary Isolation, Culture, and Growth of iSGECs
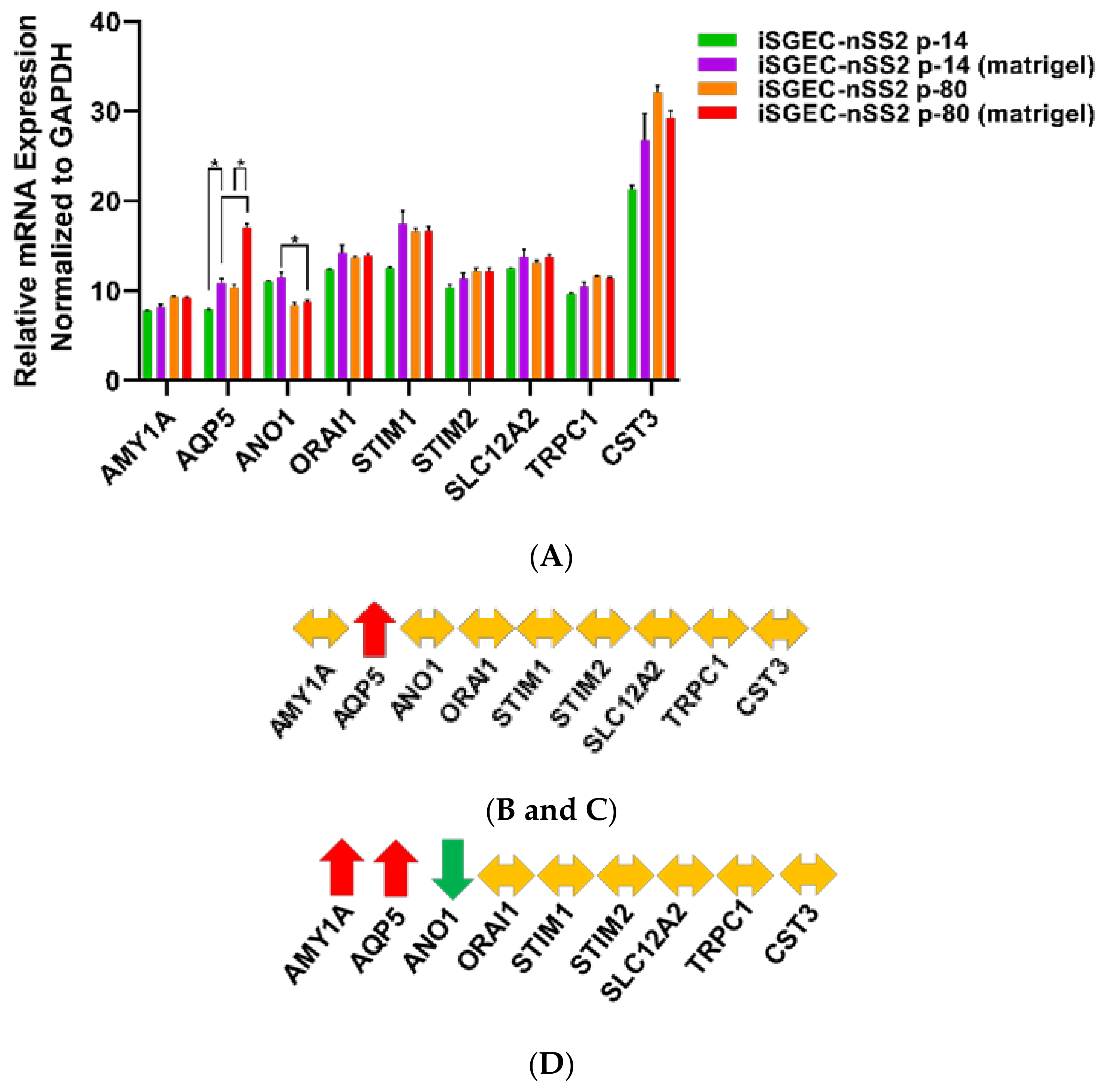
4.2. Characterization of iSGECs by qRT-PCR
4.3. Changes in mRNA Expression among Early and Late Passage iSGECs by qRT-PCR
4.4. Effects of Ca2+ on iSGECs mRNA Expression
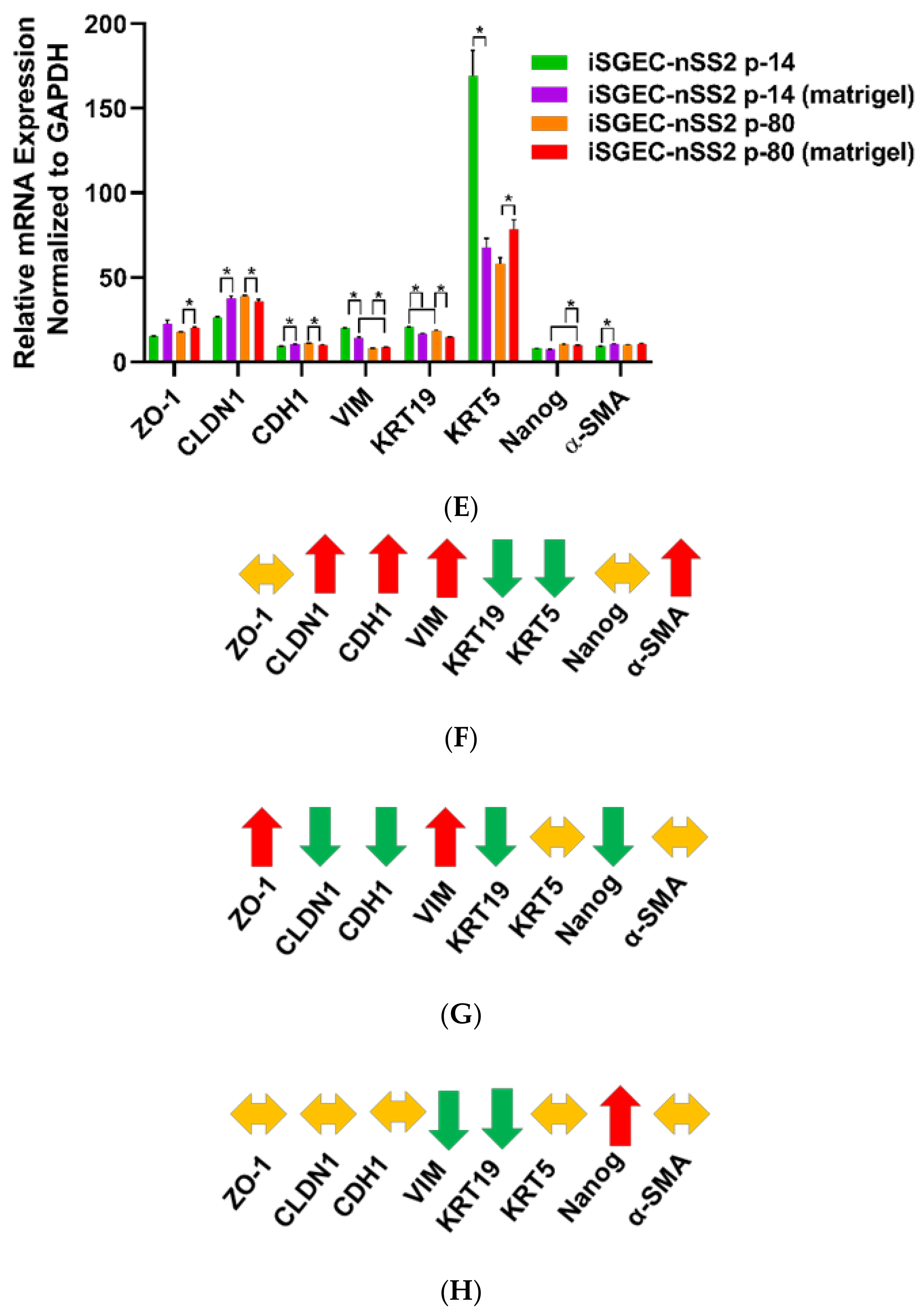
4.5. Characterization of iSGECs by ICC and Western Blot
4.5.1. ICC and Western Blot of iSGECs in Monolayer Culture
4.5.2. 3D-Matrigel Spheroid Culture
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guchelaar, H.J.; Vermes, A.; Meerwaldt, J.H. Radiation-induced xerostomia: Pathophysiology, clinical course and supportive treatment. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 1997, 5, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Ship, J.A.; Wu, A.J. Salivary gland function and aging: A model for studying the interaction of aging and systemic disease. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1992, 4, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Pringle, S.; Wang, X.; Verstappen, G.; Terpstra, J.H.; Zhang, C.K.; He, A.; Patel, V.; Jones, R.E.; Baird, D.M.; Spijkervet, F.K.L.; et al. Salivary Gland Stem Cells Age Prematurely in Primary Sjogren’s Syndrome. Arthritis Rheumatol. 2019, 71, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Elkashty, O.; Ramamoorthi, M.; Trinh, N.; Liu, Y.; Sunavala-Dossabhoy, G.; Pranzatelli, T.; Michael, D.G.; Chivasso, C.; Perret, J.; et al. Cross-contamination of the human salivary gland HSG cell line with HeLa cells: A STR analysis study. Oral Dis. 2018, 24, 1477–1483. [Google Scholar] [CrossRef]
- Jang, S.I.; Ong, H.L.; Gallo, A.; Liu, X.; Illei, G.; Alevizos, I. Establishment of functional acinar-like cultures from human salivary glands. J. Dent. Res. 2015, 94, 304–311. [Google Scholar] [CrossRef]
- Azuma, M.; Tamatani, T.; Kasai, Y.; Sato, M. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori-mutant deoxyribonucleic acid. Lab. Investig. J. Tech. Methods Pathol. 1993, 69, 24–42. [Google Scholar]
- Shirasuna, K.; Sato, M.; Miyazaki, T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer 1981, 48, 745–752. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors2. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef]
- Nam, H.; Kim, J.-H.; Hwang, J.-Y.; Kim, G.-H.; Kim, J.-W.; Jang, M.; Lee, J.-H.; Park, K.; Lee, G. Characterization of Primary Epithelial Cells Derived from Human Salivary Gland Contributing to in vivo Formation of Acini-like Structures. Mol. Cells 2018, 41, 515–522. [Google Scholar] [CrossRef]
- Dimitriou, I.D.; Kapsogeorgou, E.K.; Abu-Helu, R.F.; Moutsopoulos, H.M.; Manoussakis, M.N. Establishment of a convenient system for the long-term culture and study of non-neoplastic human salivary gland epithelial cells. Eur. J. Oral Sci. 2002, 110, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, T.; Sawaki, T.; Sakai, T.; Miki, M.; Iwao, H.; Nakajima, A.; Nakamura, T.; Sato, T.; Fujita, Y.; Tanaka, M.; et al. Skewed production of IL-6 and TGFβ by cultured salivary gland epithelial cells from patients with Sjögren’s syndrome. PLoS ONE 2012, 7, e45689. [Google Scholar] [CrossRef] [PubMed]
- Ittah, M.; Miceli-Richard, C.; Gottenberg, J.E.; Lavie, F.; Lazure, T.; Ba, N.; Sellam, J.; Lepajolec, C.; Mariette, X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren’s syndrome. Arthritis Res. 2006, 8, R51. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Dawson, P.; Zhang, Q.; Harris, Z.; Limesand, K.H. Administration of growth factors promotes salisphere formation from irradiated parotid salivary glands. PLoS ONE 2018, 13, e0193942. [Google Scholar] [CrossRef]
- Fujita-Yoshigaki, J. Plasticity in Differentiation of Salivary Glands: The Signaling Pathway That Induces Dedifferentiation of Parotid Acinar Cells. J. Oral Biosci. 2010, 52, 65–71. [Google Scholar] [CrossRef]
- Fujita-Yoshigaki, J.; Matsuki-Fukushima, M.; Sugiya, H. Inhibition of Src and p38 MAP kinases suppresses the change of claudin expression induced on dedifferentiation of primary cultured parotid acinar cells. Am. J. Physiol. Cell Physiol. 2008, 294, C774–C785. [Google Scholar] [CrossRef]
- Ahuja, D.; Sáenz-Robles, M.T.; Pipas, J.M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005, 24, 7729–7745. [Google Scholar] [CrossRef]
- Satyanarayana, A.; Greenberg, R.A.; Schaetzlein, S.; Buer, J.; Masutomi, K.; Hahn, W.C.; Zimmermann, S.; Martens, U.; Manns, M.P.; Rudolph, K.L. Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol. Cell. Biol. 2004, 24, 5459–5474. [Google Scholar] [CrossRef]
- ATCC. A-253 (ATCC® HTB-41™). Available online: https://www.atcc.org/Global/Products/D/9/3/3/HTB-41.aspx (accessed on 24 November 2020).
- Warner, K.A.; Adams, A.; Bernardi, L.; Nor, C.; Finkel, K.A.; Zhang, Z.; McLean, S.A.; Helman, J.; Wolf, G.T.; Divi, V.; et al. Characterization of tumorigenic cell lines from the recurrence and lymph node metastasis of a human salivary mucoepidermoid carcinoma. Oral Oncol. 2013, 49, 1059–1066. [Google Scholar] [CrossRef]
- Bruno, S.; Darzynkiewicz, Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, C.; Elkashty, O.A.; Chivasso, C.; Perret, J.; Tran, S.D.; Delporte, C. Insight into Salivary Gland Aquaporins. Cells 2020, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Tamma, R.; Ribatti, D.; Lisi, S. IL-6 Contributes to the TGF-β1-Mediated Epithelial to Mesenchymal Transition in Human Salivary Gland Epithelial Cells. Arch. Immunol. Ther. Exp. 2020, 68, 27. [Google Scholar] [CrossRef]
- May, A.J.; Cruz-Pacheco, N.; Emmerson, E.; Gaylord, E.A.; Seidel, K.; Nathan, S.; Muench, M.O.; Klein, O.D.; Knox, S.M. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 2018, 145, dev166363. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Ninche, N.; Klein, S.; Saur, D.; Ghazizadeh, S. c-Kit+ Cells in Adult Salivary Glands do not Function as Tissue Stem Cells. Sci. Rep. 2018, 8, 14193. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Ong, H.L.; Liu, X.; Alevizos, I.; Ambudkar, I.S. Up-regulation of Store-operated Ca2+ Entry and Nuclear Factor of Activated T Cells Promote the Acinar Phenotype of the Primary Human Salivary Gland Cells. J. Biol. Chem. 2016, 291, 8709–8720. [Google Scholar] [CrossRef]
- Pringle, S.; Wang, X.; Bootsma, H.; Spijkervet, F.K.L.; Vissink, A.; Kroese, F.G.M. Small-molecule inhibitors and the salivary gland epithelium in Sjögren’s syndrome. Expert Opin. Investig. Drugs 2019, 28, 605–616. [Google Scholar] [CrossRef]
- Draeger, A.; Nathrath, W.B.; Lane, E.B.; Sundström, B.E.; Stigbrand, T.I. Cytokeratins, smooth muscle actin and vimentin in human normal salivary gland and pleomorphic adenomas. Immunohistochemical studies with particular reference to myoepithelial and basal cells. Apmis Acta Pathol. Microbiol. Immunol. Scand. 1991, 99, 405–415. [Google Scholar] [CrossRef]
- Ikeura, K.; Kawakita, T.; Tsunoda, K.; Nakagawa, T.; Tsubota, K. Characterization of Long-Term Cultured Murine Submandibular Gland Epithelial Cells. PLoS ONE 2016, 11, e0147407. [Google Scholar] [CrossRef][Green Version]
- You, S.; Avidan, O.; Tariq, A.; Ahluwalia, I.; Stark, P.C.; Kublin, C.L.; Zoukhri, D. Role of Epithelial–Mesenchymal Transition in Repair of the Lacrimal Gland after Experimentally Induced Injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018, 131, jcs208728. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-S.; Hong, H.J.; Koh, W.-G.; Lim, J.-Y. Organotypic 3D Culture in Nanoscaffold Microwells Supports Salivary Gland Stem-Cell-Based Organization. ACS Biomater. Sci. Eng. 2018, 4, 4311–4320. [Google Scholar] [CrossRef]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. matrigel improves functional properties of primary human salivary gland cells. Tissue Eng. Part A 2011, 17, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Morgan, P.R.; Harrison, D.L.; Waseem, A.; Lane, E.B. Expression of keratin mRNAS and proteins in normal salivary epithelia and pleomorphic adenomas. J. Pathol. 1993, 171, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science 2010, 329, 1645. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.A.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheco, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 regulates acinar cell development in the salivary gland. eLife 2017, 6, e26620. [Google Scholar] [CrossRef]
- Min, S.; Song, E.-A.C.; Oyelakin, A.; Gluck, C.; Smalley, K.; Romano, R.-A. Functional characterization and genomic studies of a novel murine submandibular gland epithelial cell line. PLoS ONE 2018, 13, e0192775. [Google Scholar] [CrossRef]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of Salivary Gland Function after Stem Cell Transplantation in Irradiated Glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef]
- Weng, P.-L.; Aure, M.H.; Maruyama, T.; Ovitt, C.E. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep. 2018, 24, 1464–1470.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yan, Z.; Pei, M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; Altemani, A.; Hermsen, M. Current Concepts on Dedifferentiation/High-Grade Transformation in Salivary Gland Tumors. Pathol. Res. Int. 2011, 2011, 325965. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Billings, M.E.; Goldsmith, C.M.; Tandon, M.; Helmerhorst, E.J.; Catalan, M.A.; et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene 2017, 24, 176–186. [Google Scholar] [CrossRef]
- Baker, O.J. Tight junctions in salivary epithelium. J. Biomed. Biotechnol. 2010, 2010, 278948. [Google Scholar] [CrossRef]
- Shubin, A.D.; Sharipol, A.; Felong, T.J.; Weng, P.-L.; Schutrum, B.E.; Joe, D.S.; Aure, M.H.; Benoit, D.S.W.; Ovitt, C.E. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res. 2020, 380, 487–497. [Google Scholar] [CrossRef]
- Noll, B.; Mougeot, F.B.; Brennan, M.T.; Mougeot, J.C. Telomere erosion in Sjögren’s syndrome: A multi-tissue comparative analysis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2020, 49, 63–71. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawakita, T.; Maida, Y.; Kamoi, M.; Ogawa, Y.; Shimmura, S.; Masutomi, K.; Tsubota, K. Comparison of telomere length and association with progenitor cell markers in lacrimal gland between Sjogren syndrome and non-Sjogren syndrome dry eye patients. Mol. Vis. 2011, 17, 1397–1404. [Google Scholar]
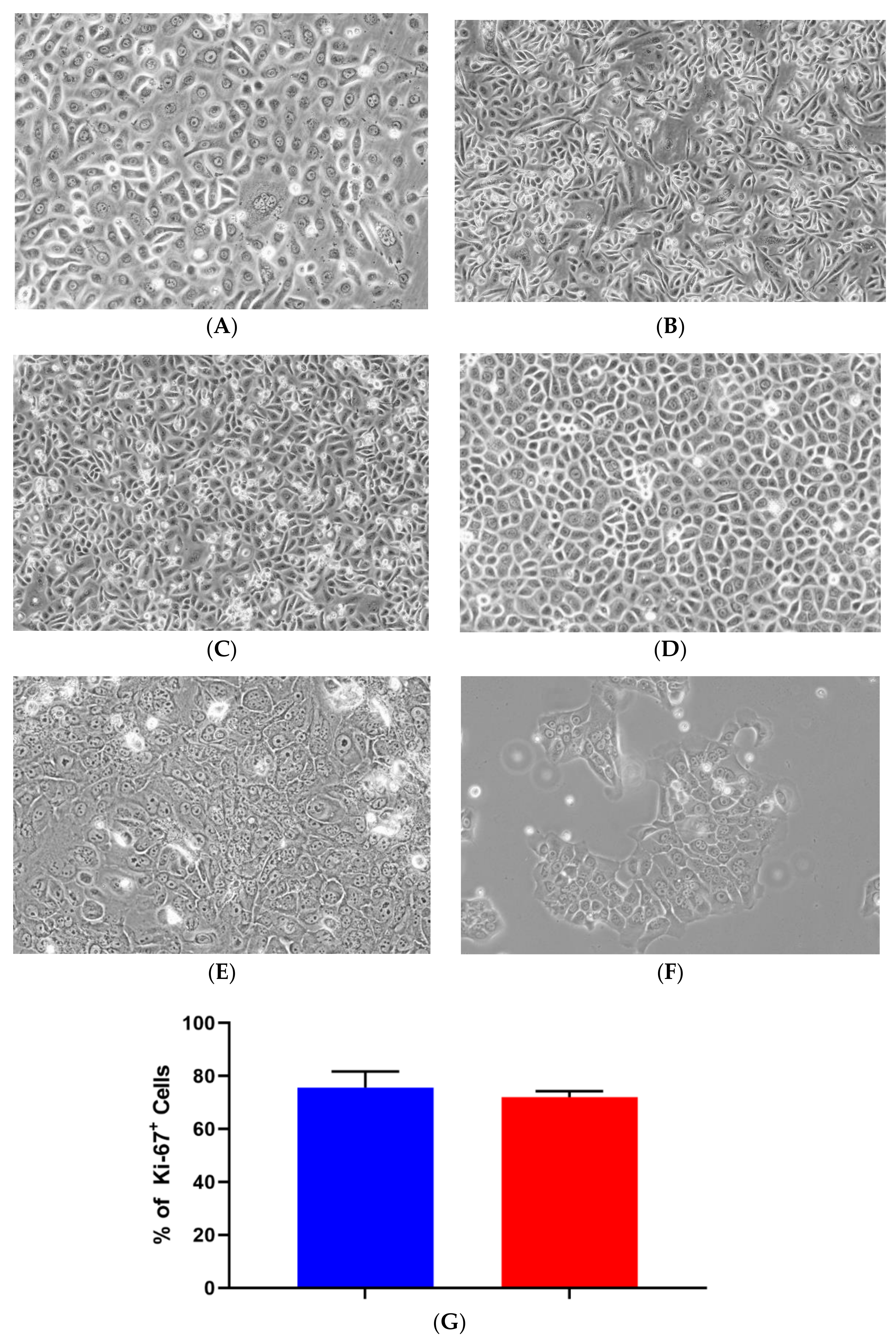


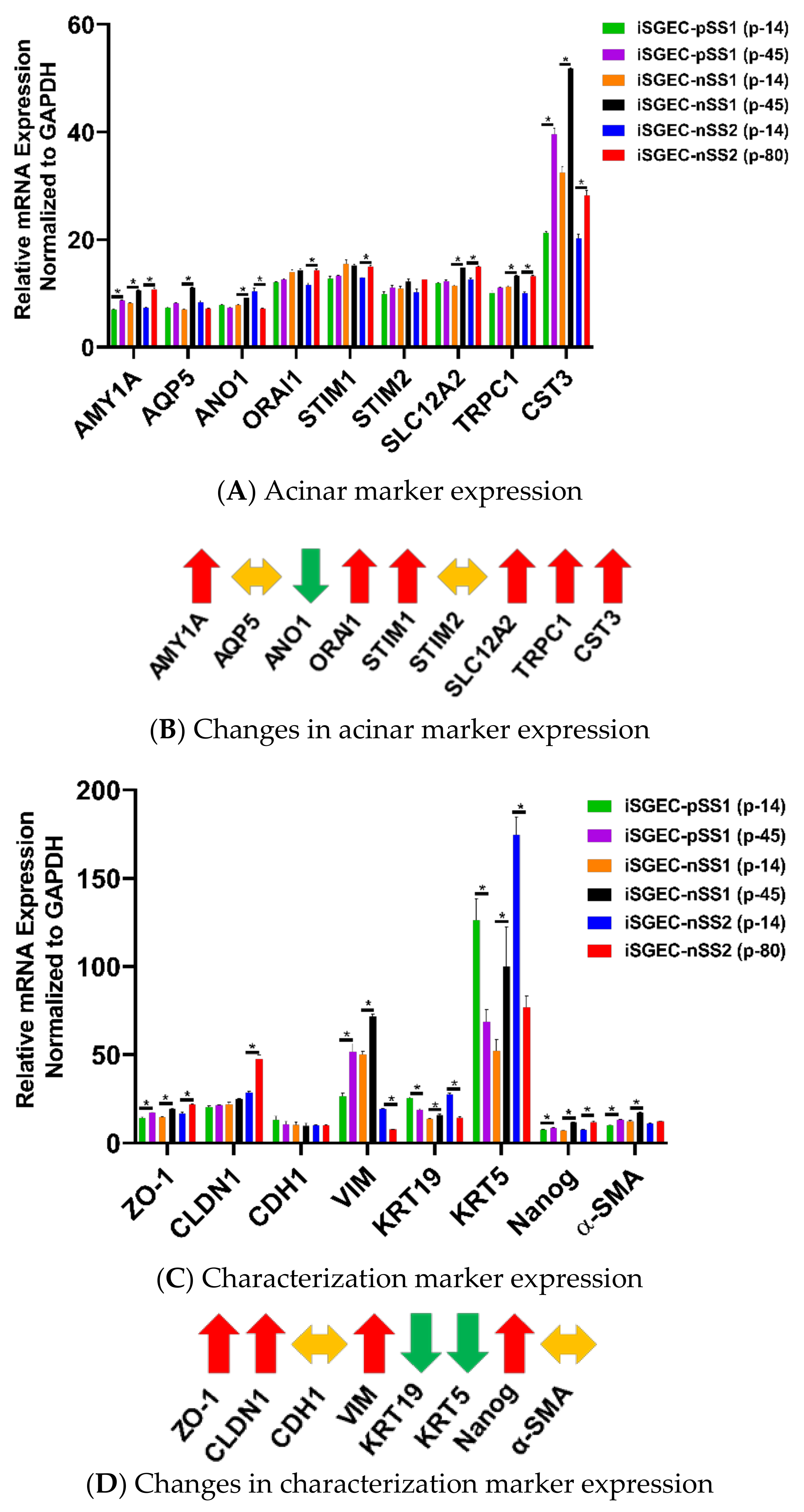
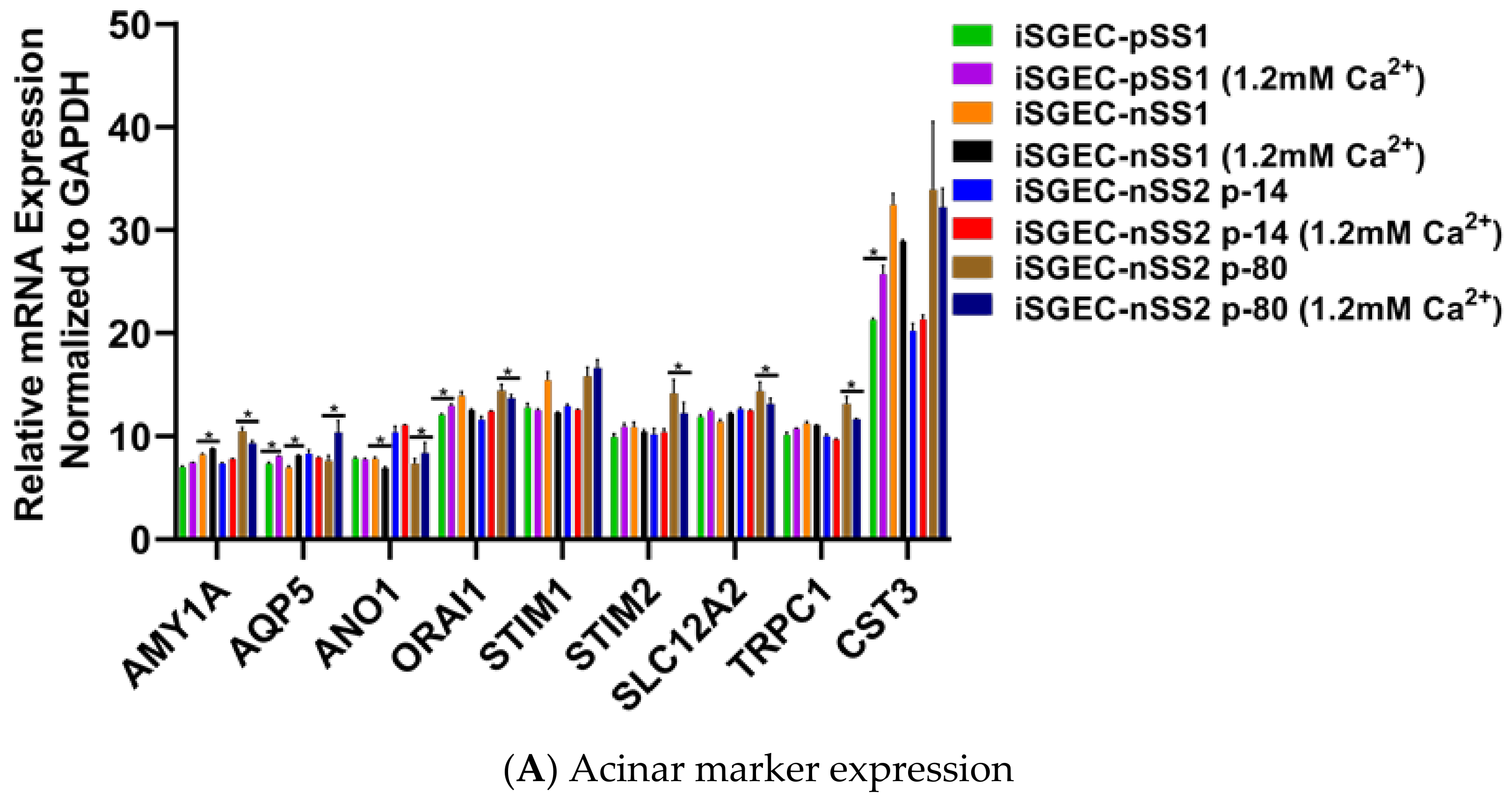

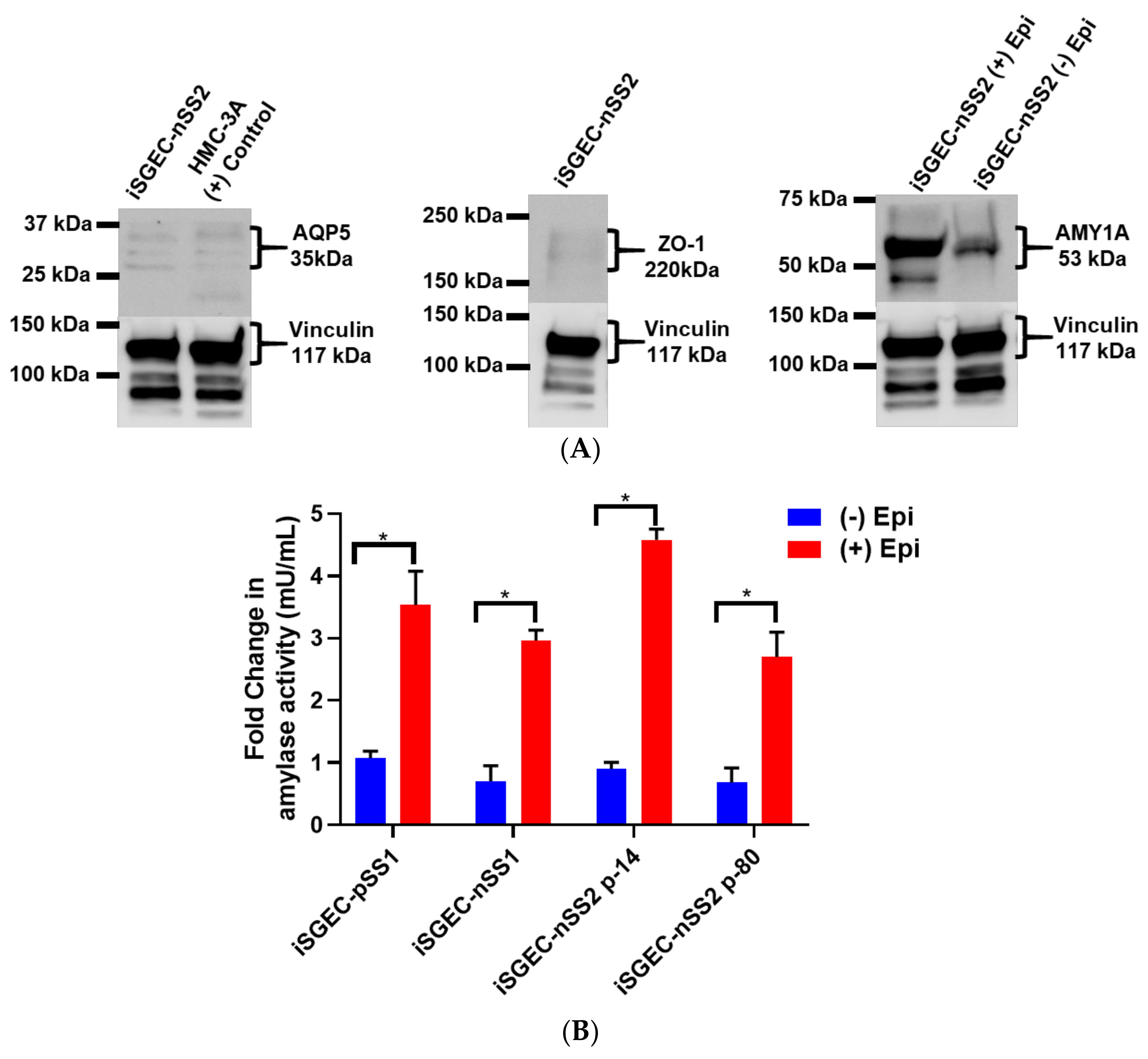

| Demographics | iSGEC-pSS1 | iSGEC-nSS1 | iSGEC-nSS2 |
|---|---|---|---|
| Age | 70 | 57 | 47 |
| Gender | Female | Female | Female |
| Race | C | C | C |
| Clinical Features | pSS | nSS | nSS |
| Focus Score | 1.8 | 0.3 | 0.16 |
| Anti-Ro (SSA) | (−) | (−) | (−) |
| Unstim. Salivary Flow (<1.5 mL/15 min) | 0.66 | 0.66 | 0.06 * |
| Stimulated Salivary Flow (mL/min) | 11.7 | 8.91 | 1.02 * |
| Schirmer’s (+/−) | NA | NA | (−) |
| DMARDs | (−) | (−) | (−) |
| Protein Target | KRT8 | K18 | K19 | ZO-1 | E-Cadherin | AQP5 | Vimentin | α-Amylase | α-SMA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line | ||||||||||||||
| iSGEC-pSS1 | + | + | + | + | + | + | + | + | + | + | + | + | + | (−/+) |
| iSGEC-nSS1 | + | + | + | (−/+) | (−/+) | + | + | + | + | + | + | + | + | (−/+) |
| iSGEC-nSS2 | + | + | + | + | + | + | + | + | + | + | + | + | + | (−) |
| A253 | + | + | + | (−/+) | + | (−/+) | + | + | + | |||||
| HMC-3A | + | + | + | (−/+) | + | + | + | + | + | |||||
| HSY | + | + | + | (−/+) | + | (−/+) | + | + | + | |||||
| HSG | + | + | + | (−/+) | + | (−/+) | + | + | + | |||||
| HeLa | + | + | + | (−/+) | (−) | (−/+) | + | (−/+) | + | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noll, B.D.; Grdzelishvili, A.; Brennan, M.T.; Mougeot, F.B.; Mougeot, J.-L.C. Immortalization of Salivary Gland Epithelial Cells of Xerostomic Patients: Establishment and Characterization of Novel Cell Lines. J. Clin. Med. 2020, 9, 3820. https://doi.org/10.3390/jcm9123820
Noll BD, Grdzelishvili A, Brennan MT, Mougeot FB, Mougeot J-LC. Immortalization of Salivary Gland Epithelial Cells of Xerostomic Patients: Establishment and Characterization of Novel Cell Lines. Journal of Clinical Medicine. 2020; 9(12):3820. https://doi.org/10.3390/jcm9123820
Chicago/Turabian StyleNoll, Braxton D., Alexandre Grdzelishvili, Michael T. Brennan, Farah Bahrani Mougeot, and Jean-Luc C. Mougeot. 2020. "Immortalization of Salivary Gland Epithelial Cells of Xerostomic Patients: Establishment and Characterization of Novel Cell Lines" Journal of Clinical Medicine 9, no. 12: 3820. https://doi.org/10.3390/jcm9123820
APA StyleNoll, B. D., Grdzelishvili, A., Brennan, M. T., Mougeot, F. B., & Mougeot, J.-L. C. (2020). Immortalization of Salivary Gland Epithelial Cells of Xerostomic Patients: Establishment and Characterization of Novel Cell Lines. Journal of Clinical Medicine, 9(12), 3820. https://doi.org/10.3390/jcm9123820





