Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Eligibility
2.2. Search Terms and Strategy
2.3. Study Selection Process
2.4. Data Extraction
2.5. Quality Assessment (Risk of Bias)
3. Results
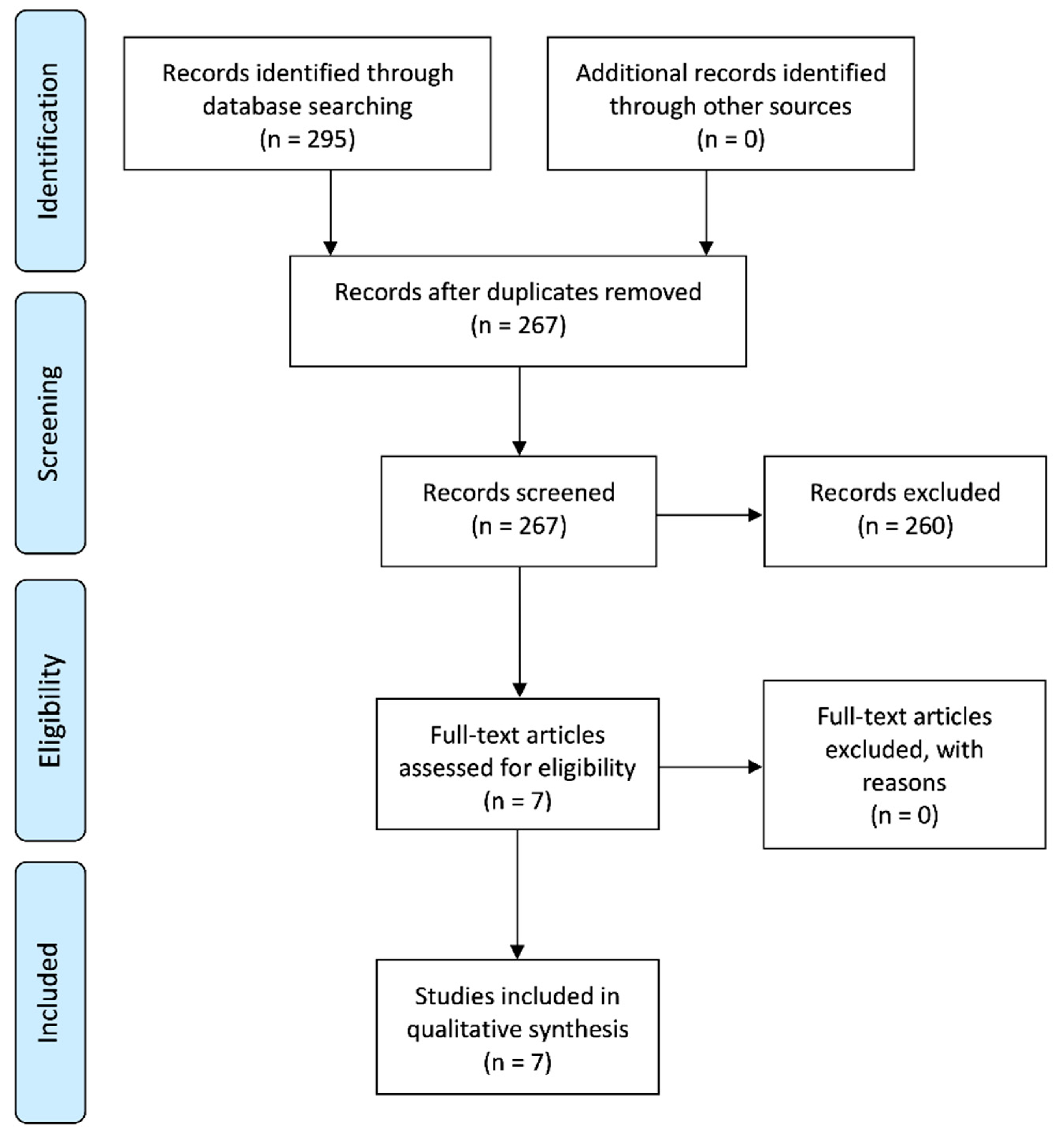
3.1. Search Results and Study Selection
3.2. Study Methodology
3.3. Study Results
3.4. Quality Assessment (Risk of Bias)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Holan, G.; Needleman, H.L. Premature loss of primary anterior teeth due to trauma-potential short- and long-term sequelae. Dent. Traumatol. 2013, 30, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, H.F.; Cooper, P.R.; Smith, A.J. Dissecting dentine–pulp injury and wound healing responses: Consequences for regenerative endodontics. Int. Endod. J. 2019, 52, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rosa, W.L.O.; Piva, E.; Da Silva, A.F. Disclosing the physiology of pulp tissue for vital pulp therapy. Int. Endod. J. 2018, 51, 829–846. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sha, X.-J.; Li, G.; Yang, F.-S.; Ji, K.; Wen, L.-Y.; Liu, S.; Chen, L.; Ding, Y.; Xuan, K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch. Oral Biol. 2012, 57, 1231–1240. [Google Scholar] [CrossRef]
- Telles, P.D.; de Andrade Moreira Machado, M.A.; Sakai, V.T.; Nör, J.E. Pulp tissue from primary teeth: New source of stem cells. J. Appl. Oral Sci. 2011, 19, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; de Andrade Moreira Machado, M.A.; Shi, S.; Santos, C.F.; Nör, J.E. SHED Differentiate into Functional Odontoblasts and Endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yamada, Y.; Nakamura, S.; Umemura, E.; Ito, K.; Ueda, M. Potential Characteristics of Stem Cells from Human Exfoliated Deciduous Teeth Compared with Bone Marrow–derived Mesenchymal Stem Cells for Mineralized Tissue-forming Cell Biology. J. Endod. 2011, 37, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V. Dental Stem Cells: Current research and future applications. Eur. J. Paediatr. Dent. 2018, 19, 257. [Google Scholar] [PubMed]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Coll, J.A.; Seale, N.S.; Vargas, K.; Marghalani, A.A.; Al Shamali, S.; Graham, L. Primary Tooth Vital Pulp Therapy: A Systematic Review and Meta-analysis. Int. J. Clin. Pediatr. Dent. 2017, 39, 16–123. [Google Scholar]
- Tomson, P.L.; Lumley, P.J.; Smith, A.J.; Cooper, P.R. Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int. Endod. J. 2016, 50, 281–292. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez-Lozano, F.J.; Llena, C.; Sauro, S.; Forner-Navarro, L. Bioactivity of Bioceramic Materials Used in the Dentin-Pulp Complex Therapy: A Systematic Review. Materials 2019, 12, 1015. [Google Scholar] [CrossRef] [Green Version]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Kim, J.R.; Nosrat, A.; Fouad, A.F. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J. Dent. 2015, 43, 241–247. [Google Scholar] [CrossRef]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Martens, L.; De Coster, P.J. Gene Expression Profiling and Molecular Signaling of Dental Pulp Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endod. 2015, 41, 1805–1817. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner-Navarro, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Dahake, P.T.; Panpaliya, N.P.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Bogar, C. Response of stem cells from human exfoliated deciduous teeth (SHED) to three bioinductive materials—An in vitro experimental study. Saudi Dent. J. 2019, 32, 43–51. [Google Scholar] [CrossRef]
- Wang, J.; Fangteng, J.-Z.; Liu, H. Effect of iRoot BP Plus on biological behavior of deciduous tooth pulp stem cells and human pulp stem cells. Shanghai Kou Qiang Yi Xue 2019, 28, 251–258. [Google Scholar]
- Athanasiadou, E.; Paschalidou, M.; Theocharidou, A.; Kontoudakis, N.; Arapostathis, K.; Bakopoulou, A. Biological interactions of a calcium silicate based cement (BiodentineTM) with Stem Cells from Human Exfoliated Deciduous teeth. Dent. Mater. 2018, 34, 1797–1813. [Google Scholar] [CrossRef]
- Araújo, L.B.; Cosme-Silva, L.; Fernandes, A.P.; De Oliveira, T.M.; Cavalcanti, B.D.N.; Gomes, J.E.; Sakai, V.T. Effects of mineral trioxide aggregate, BiodentineTM and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. J. Appl. Oral Sci. 2018, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awidi, A.; Hasweh, N.; Rajab, L.; Hiyasat, A.; Jafar, H.; Islam, N.; Hasan, M.; Abuarqoub, D. Characterization of the biological effect of BiodentineTM on primary dental pulp stem cells. Indian J. Dent. Res. 2018, 29, 787. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Ke, M.-C.; Chen, Y.-H.; Kuo, H.-K.; Yu, H.-J.; Chen, C.-T.; Tseng, Y.-C.; Chuang, P.-C.; Wu, P.-C. Mineral trioxide aggregate affects cell viability and induces apoptosis of stem cells from human exfoliated deciduous teeth. BMC Pharmacol. Toxicol. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Ortolani-Seltenerich, P.S.; Álvarez-Muro, T.; Lozano, A.; Forner, L.; Llena, C.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. Int. Endod. J. 2017, 50, e19–e30. [Google Scholar] [CrossRef] [PubMed]
- Ghilotti, J.; Sanz, J.L.; López-García, S.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodríguez-Lozano, F.J.; Forner-Navarro, L.; Spagnuolo, G. Comparative Surface Morphology, Chemical Composition, and Cytocompatibility of Bio-C Repair, Biodentine, and ProRoot MTA on hDPCs. Materials 2020, 13, 2189. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Castelo-Baz, P.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50, e63–e72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, E.M.; Cornélio, A.L.G.; Mestieri, L.B.; Fuentes, A.S.C.; Salles, L.P.; Rossa-Junior, C.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Human dental pulp cells response to mineral trioxide aggregate (MTA) and MTA Plus: Cytotoxicity and gene expression analysis. Int. Endod. J. 2017, 50, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Van Landuyt, K.; Van Meerbeek, B. Cytotoxicity and Bioactivity of Dental Pulp-Capping Agents towards Human Tooth-Pulp Cells: A Systematic Review of In-Vitro Studies and Meta-Analysis of Randomized and Controlled Clinical Trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef]
- Li, Y.; Sui, B.; Dahl, C.; Bergeron, B.; Shipman, P.; Niu, L.-N.; Chen, J.-H.; Tay, F.R. Pulpotomy for carious pulp exposures in permanent teeth: A systematic review and meta-analysis. J. Dent. 2019, 84, 1–8. [Google Scholar] [CrossRef]
- Grawish, M.E.; Mahmoud, S.H.; El-Negoly, S.A.; El-Din, A.Z.; El-Zekrid, M.H.; Grawish, L.M.; Grawish, H. Biodentine versus mineral trioxide aggregate as a direct pulp capping material for human mature permanent teeth—A systematic review. J. Conserv. Dent. 2018, 21, 466–473. [Google Scholar] [CrossRef]
- Bossù, M.; Iaculli, F.; Di Giorgio, G.; Salucci, A.; Polimeni, A.; Di Carlo, S. Different Pulp Dressing Materials for the Pulpotomy of Primary Teeth: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadros-Fernández, C.; Rodríguez, A.L.; Sáez-Martínez, S.; García-Binimelis, J.; Mercadé, M. Short-term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: A randomized clinical trial. Clin. Oral Investig. 2020, 20, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Guven, Y.; Aksakal, S.D.; Avcu, N.; Ünsal, G.; Tuna, E.B.; Aktoren, O. Success Rates of Pulpotomies in Primary Molars Using Calcium Silicate-Based Materials: A Randomized Control Trial. BioMed Res. Int. 2017, 2017, 4059703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çelik, B.N.; Mutluay, M.S.; Arıkan, V.; Sarı, Ş. The evaluation of MTA and Biodentine as a pulpotomy materials for carious exposures in primary teeth. Clin. Oral Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Pulikkotil, S.J.; Veettil, S.K.; Jinatongthai, P.; Gutmann, J.L.; Gutman, J.L. Efficacy of Biodentine and Mineral Trioxide Aggregate in Primary Molar Pulpotomies—A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials. J. Evid. Based Dent. Pract. 2019, 19, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Junior, E.S.; Dos Santos, M.G.C.; Oliveira, L.B.; Mercadé, M. MTA and biodentine for primary teeth pulpotomy: A systematic review and meta-analysis of clinical trials. Clin. Oral Investig. 2019, 23, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Moosavi, F.N.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V.; et al. Human Pulp Responses to Partial Pulpotomy Treatment with TheraCal as Compared with Biodentine and ProRoot MTA: A Clinical Trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef]
- Erfanparast, L.; Iranparvar, P.; Vafaei, A. Direct pulp capping in primary molars using a resin-modified Portland cement-based material (TheraCal) compared to MTA with 12-month follow-up: A randomised clinical trial. Eur. Arch. Paediatr. Dent. 2018, 19, 197–203. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part II: Leakage and Biocompatibility Investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Woo, S.-M.; Hwang, Y.-C.; Lim, H.-S.; Choi, N.; Kim, S.-H.; Kim, W.-J.; Kim, S.-M.; Jung, J.-Y. Effect of Nifedipine on the Differentiation of Human Dental Pulp Cells Cultured with Mineral Trioxide Aggregate. J. Endod. 2013, 39, 801–805. [Google Scholar] [CrossRef]
- Slompo, C.; Peres-Buzalaf, C.; Gasque, K.C.D.S.; Damante, C.A.; Ordinola-Zapata, R.; Duarte, M.A.H.; De Oliveira, R.C. Experimental Calcium Silicate-Based Cement with and without Zirconium Oxide Modulates Fibroblasts Viability. Braz. Dent. J. 2015, 26, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-M.; Shen, Y.; Wang, Z.; Li, L.; Zheng, Y.-F.; Häkkinen, L.; Haapasalo, M. In Vitro Cytotoxicity Evaluation of a Novel Root Repair Material. J. Endod. 2013, 39, 478–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; He, W.; Song, Z.; Tong, Z.; Li, S.; Ni, L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol. Biol. Rep. 2011, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, D.; Kohli, M.R.; Yu, Q.; Kim, S.; He, W.-X. Effect of BiodentineTM on the proliferation, migration and adhesion of human dental pulp stem cells. J. Dent. 2014, 42, 490–497. [Google Scholar] [CrossRef]
- Saberi, E.A.; Farhad-Mollashahi, N.; Aval, F.S.; Saberi, M. Proliferation, odontogenic/osteogenic differentiation, and cytokine production by human stem cells of the apical papilla induced by biomaterials: A comparative study. Clin. Cosmet. Investig. Dent. 2019, 11, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Hanafy, A.K.; Shinaishin, S.F.; Eldeen, G.N.; Aly, R.M. Nano Hydroxyapatite & Mineral Trioxide Aggregate Efficiently Promote Odontogenic Differentiation of Dental Pulp Stem Cells. Open Access Maced. J. Med. Sci. 2018, 6, 1727–1731. [Google Scholar]
- Pedano, M.S.; Li, X.; Li, S.; Sun, Z.; Cokic, S.M.; Putzeys, E.; Yoshihara, K.; Yoshida, Y.; Chen, Z.; Van Landuyt, K.; et al. Freshly-mixed and setting calcium-silicate cements stimulate human dental pulp cells. Dent. Mater. 2018, 34, 797–808. [Google Scholar] [CrossRef]
- Kang, K.-J.; Lee, M.S.; Moon, C.-W.; Lee, J.-H.; Yang, H.S.; Jang, Y.-J. In Vitro and In Vivo Dentinogenic Efficacy of Human Dental Pulp-Derived Cells Induced by Demineralized Dentin Matrix and HA-TCP. Stem Cells Int. 2017, 2017, 2416254. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.L.; Forner-Navarro, L.; Almudéver, A.; Guerrero-Gironés, J.; Llena, C. Viability and Stimulation of Human Stem Cells from the Apical Papilla (hSCAPs) Induced by Silicate-Based Materials for Their Potential Use in Regenerative Endodontics: A Systematic Review. Materials 2020, 13, 974. [Google Scholar] [CrossRef] [Green Version]
- Winning, L.; El Karim, I.A.; Lundy, F.T. A Comparative Analysis of the Osteogenic Potential of Dental Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef]
- Chadipiralla, K.; Yochim, J.M.; Bahuleyan, B.; Huang, C.-Y.C.; García-Godoy, F.; Murray, P.E.; Stelnicki, E.J. Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res. 2010, 340, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; Bueno, C.R.; Insausti, C.L.; Meseguer, L.; Ramírez, M.C.; Blanquer, M.; Marín, N.; Martínez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Database | Search Strategy | Findings |
|---|---|---|
| Medline | #1 (silicate) OR (bioceramic) | 47,275 |
| #2 (stem cells from human exfoliated deciduous teeth) OR (SHED) | 60,545 | |
| #3 (((((cytocompatibility) OR (biocompatibility)) OR (bioactivity)) OR (differentiation)) OR (expression)) OR (mineralization) | 4,715,994 | |
| #1 AND #2 AND #3 | 67 | |
| Scopus | #1 ALL (silicate OR bioceramic) | 508,929 |
| #2 ALL (stem cells from human exfoliated deciduous teeth OR SHED) | 2972 | |
| #3 ALL (cytocompatibility OR biocompatibility OR bioactivity OR differentiation OR expression OR mineralization) | 9,035,995 | |
| #1 AND #2 AND #3 | 178 | |
| Embase | #1 (silicate OR bioceramic) | 24,472 |
| #2 (stem cells from human exfoliated deciduous teeth OR SHED) | 72,196 | |
| #3 (cytocompatibility OR biocompatibility OR bioactivity OR differentiation OR expression OR mineralization) | 4,096,064 | |
| #1 AND #2 AND #3 | 11 | |
| Web of Science | #1 TS = (silicate OR bioceramic) | 108,951 |
| #2 TS = (stem cells from human exfoliated deciduous teeth OR SHED) | 185,496 | |
| #3 TS = (cytocompatibility OR biocompatibility OR bioactivity OR differentiation OR expression OR mineralization) | 4,004,895 | |
| #1 AND #2 AND #3 | 39 | |
| SciELO | #1 (silicate OR bioceramic) | 675 |
| #2 (stem cells from human exfoliated deciduous teeth OR SHED) | 12 | |
| #3 (cytocompatibility OR biocompatibility OR bioactivity OR differentiation OR expression OR mineralization) | 21,081 | |
| #1 AND #2 AND #3 | 0 |
| Author | Cell Variant | Materials Used | Control Groups | Activity Analysis | Duration |
|---|---|---|---|---|---|
| Dahake et al., 2020 [28] | SHED (8–12 years), 5th passage | MTA (1 mg/mL), BD (1 mg/mL), | Negative: SHED + DMEM, 10% FBS, 2 mmol/L L-glutamine, 1% penicillin, streptomycin, and amphotericin (PSA); Positive: SHED + DMEM, 20% FBS, 50 μg/mL ascorbic acid, 50n mol/L β glycerol phosphate, 10–8 mol/L dexamethasone. | MTT Assay | 7 days |
| ARS | 14 days | ||||
| Wang et al., 2019 [29] | SHED (6–10 years), 4–6th passage | iRoot BP (n/s), MTA (n/s) | Negative: SHED + αMEM, 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin. | CCK8 | 1,3,5,7 days |
| Transwell migration assay | 24 h | ||||
| Wound healing assay | 24 h | ||||
| Immunofluorescence staining | 1,3,5 days | ||||
| ALP activity assay | 7,14 days | ||||
| ARS | 21 days | ||||
| Athanasiadou et al., 2018 [30] | SHED (3–10 years), 2–6th passage | BD (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128 eluates) | Negative: SHED + αMEM, 15% FBS, 100 μM L-ascorbic acid phosphate, 100 U/mL penicillin, 100 mg/mL streptomycin, 0.25 mg/mL Amphotericin B; Positive: negative + 0.01 mM dexamethasone disodium phosphate, 1.8 mM KH2PO4, 5 mM b-glycerophosphate. | MTT Assay | 24, 72, 120 h |
| SEM | 72 h | ||||
| Live/dead fluorescent staining | 72 h | ||||
| qRT-PCR | 7,14 days | ||||
| ARS | 14 days | ||||
| Araújo et al., 2018 [31] | SHED (7–8 years), 4–8th passage | MTA (1 mg/mL), BD (1 mg/mL), | Negative: SHED + αMEM, 10%FBS, 1% penicillin-streptomycin; Positive: SHED + αMEM, 20% FBS, 1% penicillin-streptomycin. | MTT assay | 1,3,5,7 days |
| SRB assay | 1,3,5,7days | ||||
| Cell migration assay | Overnight | ||||
| qRT-PCR | 1,7,14,21days | ||||
| Awidi et al., 2018 [32] | SHED (5–6 years), 3rd passage | BD (0.02 mg/mL, 0.2 mg/mL, 2 mg/mL, 20 mg/mL) | Negative: SHED + αMEM, 5% platelet lysate. | MTT assay | 6 days |
| Wound healing assay | 24 h | ||||
| Transwell migration assay | 24 h | ||||
| Cell adhesion assay | 1 h | ||||
| Tsai et al., 2018 [33] | SHED (5–7 years), 3–4th passage | 1g PR MTA:5 ml dd H2O | Negative: SHED + αMEM, 15% FBS, 100 μM L-ascorbic acid phosphate, 2 mM L-glutamine, 100 U of antibiotic-antimycotic. | Wst-1 assay | 1,2,3 days |
| Annexin-V/7-AAD staining | 2 days | ||||
| Collado-González et al., 2017 [34] | SHED (6–9 years), n/s | 1:1, 1:2 and 1:4 of: BD, MTA, Theracal, IRM | Negative: DMEM + penicillin-streptomycin. | MTT assay | 24,48,72 h |
| Annexin-V/7-AAD staining | 72h | ||||
| Cell migration assay | 24,48 h | ||||
| SEM | 3 days | ||||
| ARS | 7,14,21 days |
| Author | Assay | Significant Results | Duration | p Value |
|---|---|---|---|---|
| Dahake et al., 2020 [28] | MTT assay | BD > MTA > -control | 7 days | p < 0.001 |
| Wang et al., 2019 [29] | CCK8 | iRoot BP, MTA > -control | 7 days | p < 0.05 |
| iRoot BP > MTA > -control | 3,5 days | p < 0.05 | ||
| Transwell migration assay | iRoot BP > MTA > -control | 24 h | p < 0.05 | |
| Athanasiadou et al., 2018 [30] | MTT assay | 1:16, 1:32, 1:64 BD > -control | 72 h | p < 0.05 |
| Araújo et al., 2018 [31] | MTT assay | +control > MTA, BD, -control | 3,5 days | p <0.05 |
| MTA > BD, -control | 7 days | p < 0.05 | ||
| SRB assay | +control > MTA, BD | 1,3,5,7 days | p < 0.05 | |
| BD > MTA | 3,5 days | p < 0.05 | ||
| Cell migration assay | BD, MTA > -control | Overnight | p < 0.005 | |
| Awidi et al., 2018 [32] | MTT assay | 0.02, 0.2, 2 mg/mL > 20 mg/mL | 6 days | p < 0.0001 |
| Transwell migration assay | 0.02, 0.2, 2 mg/mL > -control | 24 h | p < 0.0037 | |
| Tsai et al., 2018 [33] | Wst-1 assay | -control DC > PR MTA DC | 1 days | p < 0.0001 |
| 2 days | p < 0.01 | |||
| 3 days | p < 0.05 | |||
| -control IDC > PR MTA IDC | 1 days | p < 0.05 | ||
| 3 days | p < 0.01 | |||
| Collado-González et al., 2017 [34] | MTT assay | MTA > -control | 48,72 h | p < 0.01 |
| BD > -control | 48,72 h | p < 0.001 | ||
| BD > MTA | 48,72 h | p < 0.01 | ||
| -control > Theracal | 24,48,72 h | p < 0.001 | ||
| Cell migration assay | BD > -control | 48 h | p < 0.001 | |
| MTA > -control | 48 h | p < 0.001 | ||
| -control > Theracal | 48 h | p < 0.001 |
| Author | Assay | Significant Results | Duration | p Value |
|---|---|---|---|---|
| Dahake et al., 2020 [28] | ARS | +control > BD > MTA > -control | 14 days | p < 0.001 |
| Wang et al., 2019 [29] | ALPs | iRoot BP > MTA > -control | 7,14 days | p < 0.05 |
| ARS | iRoot BP > MTA > -control | 21 days | p < 0.05 | |
| Athanasiadou et al., 2018 [30] | ARS | 1:4, 1:8, 1:16, 1:32 BD > -control; +control > 1:1, 1:2, 1:4, 1:8, 1:16 BD | 14 days | p < 0.05 |
| Collado-González et al., 2017 [34] | ARS | BD > -control | 7 days | p < 0.01 |
| 14 days | p < 0.05 | |||
| 21 days | p < 0.001 | |||
| -control > Theracal | 7, 21 days | p < 0.01 | ||
| 14 days | p < 0.001 |
| Studies | Modified CONSORT Checklist | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | 2b | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | % | |
| Dahake et al., 2020 [28] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | Y | N | 57 |
| Wang et al., 2019 [29] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | 64 |
| Athanasiadou et al., 2018 [30] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | 64 |
| Araújo et al., 2018 [31] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | Y | Y | N | 64 |
| Awidi et al., 2018 [32] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | Y | N | 57 |
| Tsai et al., 2018 [33] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | Y | N | 57 |
| Collado-González et al., 2017 [34] | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | Y | N | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, J.L.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Melo, M.; Rengo, S.; Spagnuolo, G.; Rodríguez-Lozano, F.J. Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies. J. Clin. Med. 2020, 9, 3872. https://doi.org/10.3390/jcm9123872
Sanz JL, Forner L, Llena C, Guerrero-Gironés J, Melo M, Rengo S, Spagnuolo G, Rodríguez-Lozano FJ. Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies. Journal of Clinical Medicine. 2020; 9(12):3872. https://doi.org/10.3390/jcm9123872
Chicago/Turabian StyleSanz, José Luis, Leopoldo Forner, Carmen Llena, Julia Guerrero-Gironés, María Melo, Sandro Rengo, Gianrico Spagnuolo, and Francisco Javier Rodríguez-Lozano. 2020. "Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies" Journal of Clinical Medicine 9, no. 12: 3872. https://doi.org/10.3390/jcm9123872
APA StyleSanz, J. L., Forner, L., Llena, C., Guerrero-Gironés, J., Melo, M., Rengo, S., Spagnuolo, G., & Rodríguez-Lozano, F. J. (2020). Cytocompatibility and Bioactive Properties of Hydraulic Calcium Silicate-Based Cements (HCSCs) on Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs): A Systematic Review of In Vitro Studies. Journal of Clinical Medicine, 9(12), 3872. https://doi.org/10.3390/jcm9123872








