Postoperative Trends of Serum C-Reactive Protein Levels after Primary Shoulder Arthroplasty—Normal Trajectory and Influencing Factors
Abstract
1. Introduction
2. Patients and Methods
2.1. Data Collection
2.2. Surgical Treatment
2.3. Laboratory Work
2.4. Statistical Analysis
3. Results
3.1. Demographics
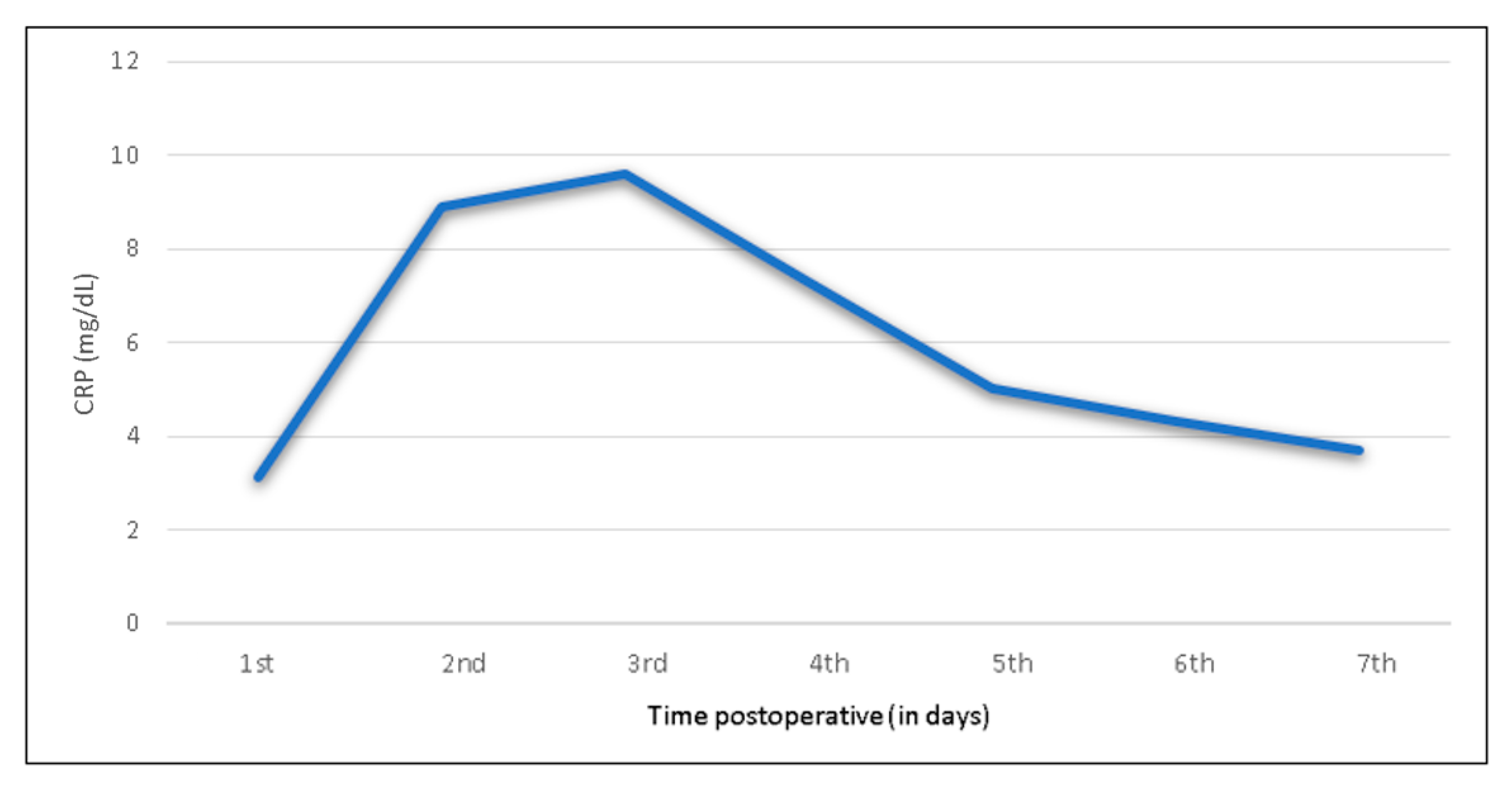
3.2. CRP Trend after Shoulder Arthroplasty
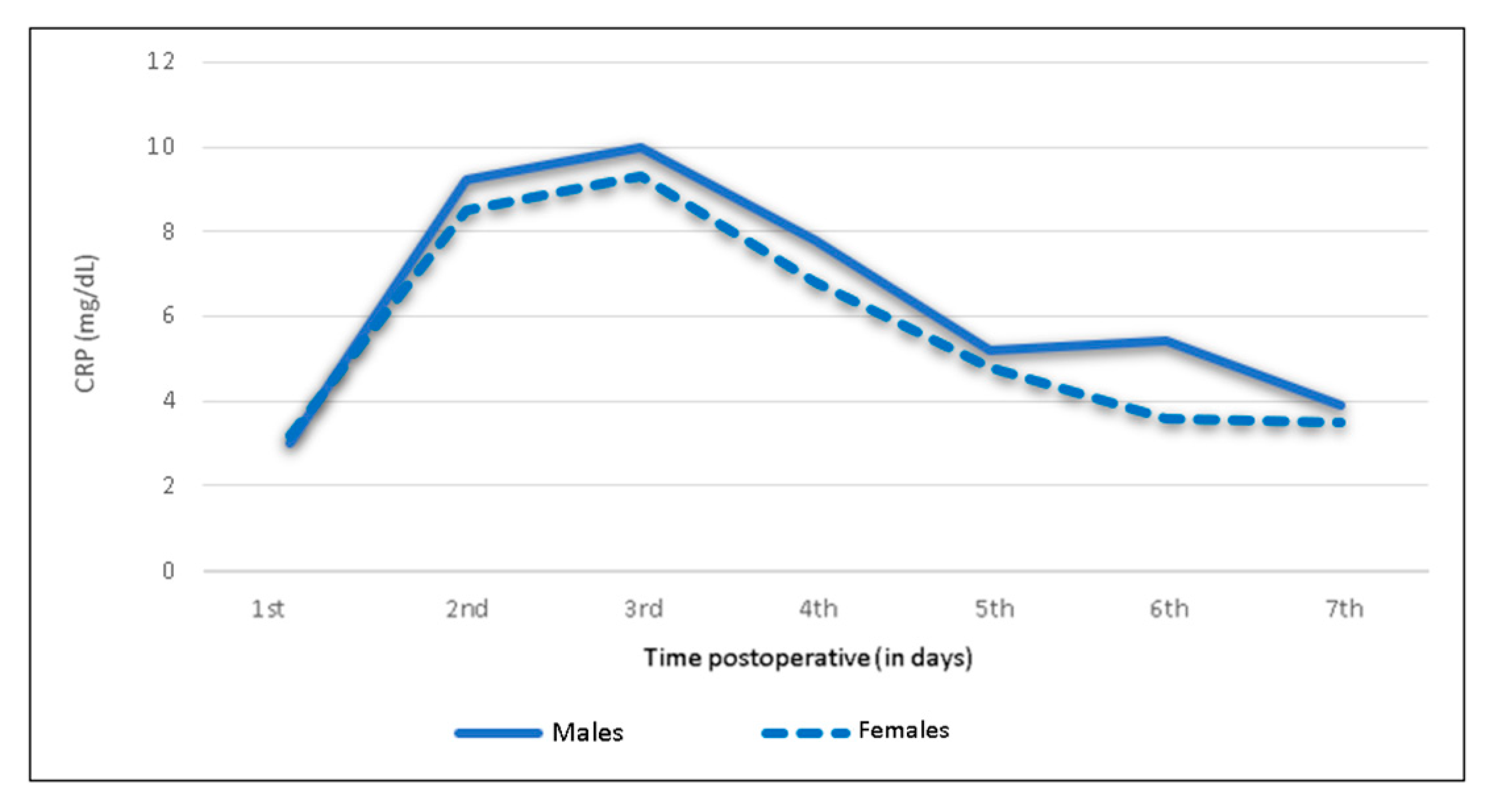
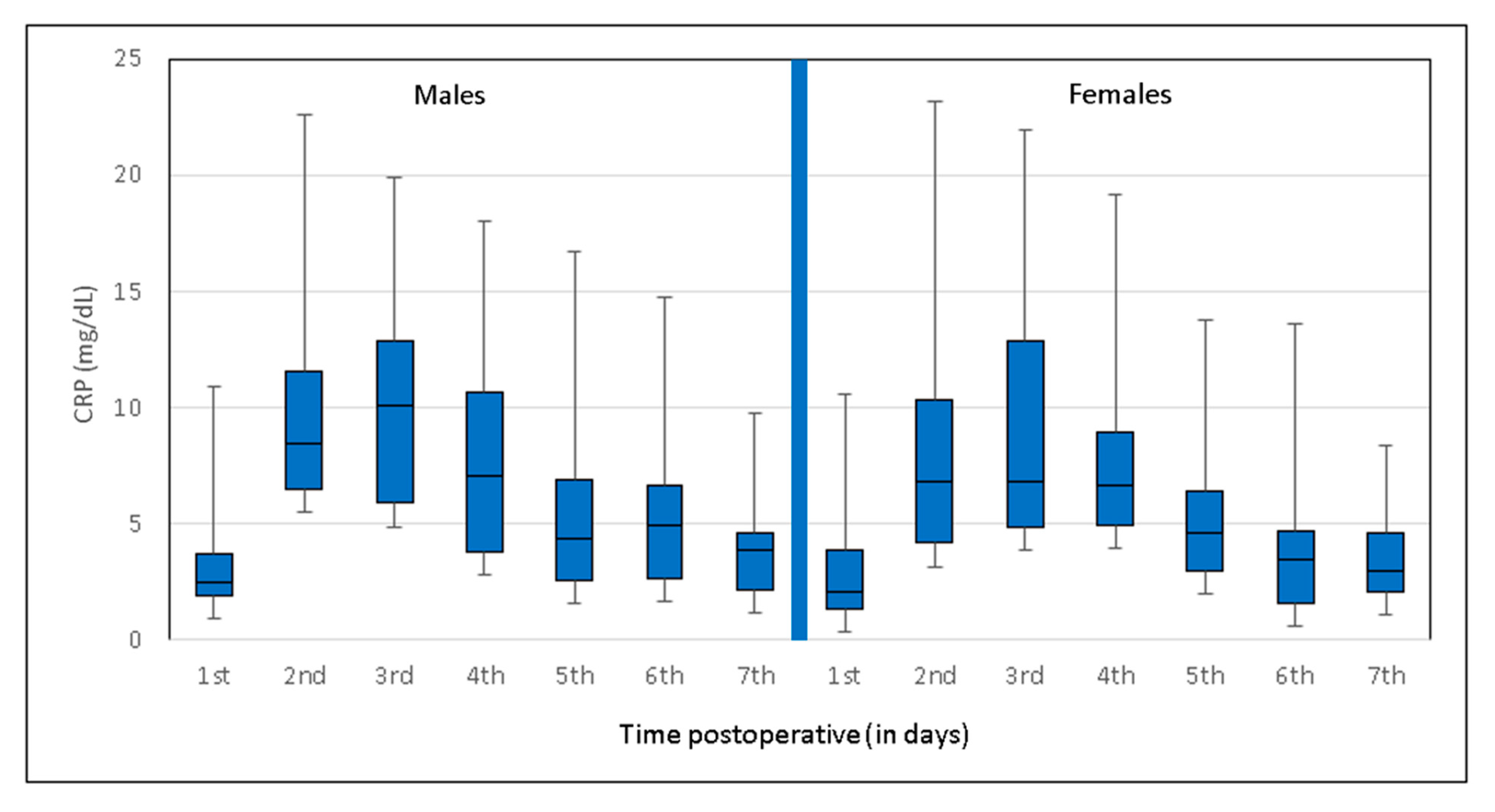
3.3. Sex-Specific Postoperative CRP Trend
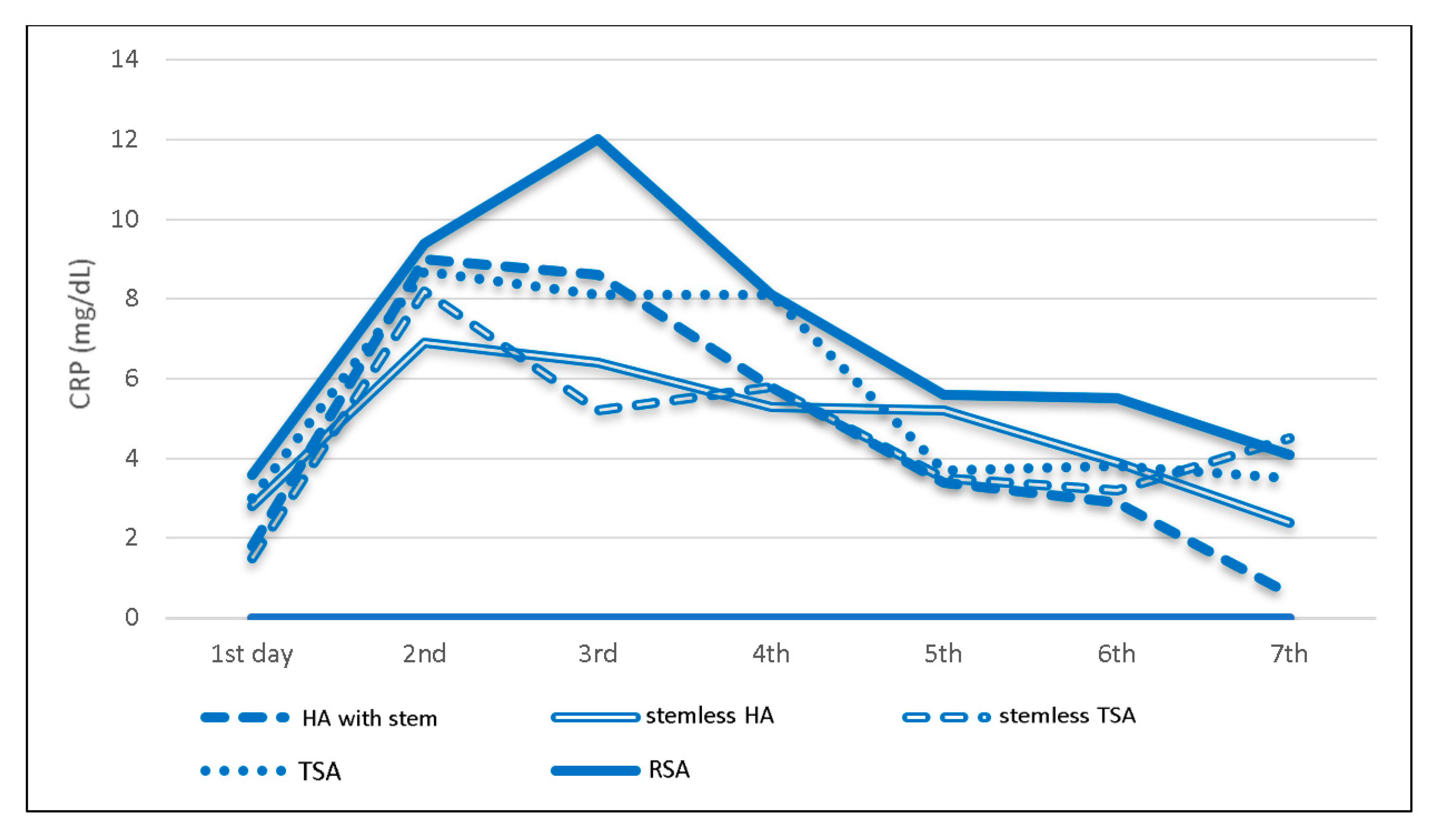
3.4. Prosthesis-Specific Postoperative CRP Trend
3.5. Influence of BMI, Operating Time and Humeral Cement Fixation
4. Discussion
4.1. Physiological CRP Kinetics and Clinical Application in Orthopedics
4.2. CRP Kinetics Following Shoulder Arthroplasty
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CRP | C-reactive protein |
| HA | Hemiarthroplasty |
| IQR | Interquartile range |
| PJI | Periprosthetic joint infection |
| PMMA | Polymethylmethacrylate (bone cement) |
| RSA | Reverse shoulder arthroplasty |
| SA | Shoulder arthroplasty |
| THA | Total hip arthroplasty |
| TKA | Total knee arthroplasty |
| TSA | Total shoulder arthroplasty |
References
- Neumaier, M.; Metak, G.; Scherer, M.A. C-reactive protein as a parameter of surgical trauma: CRP response after different types of surgery in 349 hip fractures. Acta Orthop. 2006, 77, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, S.; Fortina, M.; Carta, S.; Guerranti, R.; Nobile, F.; Ferrata, P. Serum C-Reactive Protein and Procalcitonin Kinetics in Patients Undergoing Elective Total Hip Arthroplasty. BioMed Res. Int. 2014, 1–6. Available online: https://www.hindawi.com/journals/bmri/2014/565080/citations/. (accessed on 21 December 2017). [CrossRef] [PubMed]
- Choudhry, R.; Rice, R.; Triffitt, P.; Harper, W.; Gregg, P. Plasma viscosity and C-reactive protein after total hip and knee arthroplasty. J. Bone Jt. Surg. Br. 1992, 74, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Alijanipour, P.; Bakhshi, H.; Parvizi, J. Diagnosis of Periprosthetic Joint Infection: The Threshold for Serological Markers. Clin. Orthop. Relat. Res. 2013, 471, 3186–3195. [Google Scholar] [CrossRef]
- Bonnevialle, N.; Dauzères, F.; Toulemonde, J.; Elia, F.; Laffosse, J.-M.; Mansat, P. Periprosthetic shoulder infection: An overview. EFORT Open Rev. 2017, 2, 104–109. [Google Scholar] [CrossRef]
- White, J.; Kelly, M.; Dunsmuir, R. C-reactive protein level after total hip and total knee replacement. J. Bone Jt. Surg. Br. 1998, 80-B, 909–911. [Google Scholar] [CrossRef]
- Larsson, S.M.D.; Thelander, U.M.D.; Friberg, S.M.D. C-Reactive Protein (CRP) Levels After Elective Orthopedic Surgery. Clin. Orthop. Relat. Res. 1992, 275, 237–242. [Google Scholar] [CrossRef]
- Torrens, C.; Santana, F.; Marí, R.; Puig, L.; Alier, A. Serum C-reactive protein in patients undergoing elective shoulder arthroplasty. Prospective study. J. Orthop. Sci. 2017, 22, 858–861. [Google Scholar] [CrossRef]
- Coste, J.S.; Reig, S.; Trojani, C.; Berg, M.; Walch, G.; Boileau, P. The management of infection in arthroplasty of the shoulder. J. Bone Jt. Surg. Br. 2004, 86, 65–69. [Google Scholar] [CrossRef]
- Saleh, A.; George, J.; Faour, M.; Klika, A.K.; Higuera, C.A. Serum biomarkers in periprosthetic joint infections. Bone Jt. Res. 2018, 7, 85–93. [Google Scholar] [CrossRef]
- Kragsbjerg, P.; Holmberg, H.; Vikerfors, T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur. J. Surg. Acta Chir. 1995, 161, 17–22. [Google Scholar]
- Ellitsgaard, N.; Andersson, A.; Jensen, K.; Jorgensen, M. Changes in C-reactive protein and erythrocyte sedimentation rate after hip fractures. Int. Orthop. 1991, 15, 311–314. [Google Scholar] [CrossRef]
- Lim, S.-J.; Choi, K.-H.; Lee, J.H.; Jung, J.Y.; Han, W.; Lee, B.H. Different Kinetics of Perioperative CRP after Hip Arthroplasty for Elderly Femoral Neck Fracture with Elevated Preoperative CRP. BioMed Res. Int. 2018, 1–9. [Google Scholar] [CrossRef]
- Niskanen, R.O.; Korkala, O.; Pammo, H. Serum C-reactive protein levels after total hip and knee arthroplasty. J. Bone Jt. Surg. Br. 1996, 78, 431–433. [Google Scholar] [CrossRef]
- Thienpont, E.; Grosu, I.; Jonckheere, S.; Yombi, J.C. C-reactive protein (CRP) in different types of minimally invasive knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2012, 21, 2603–2610. [Google Scholar] [CrossRef]
- Windisch, C.; Brodt, S.; Roehner, E.; Matziolis, G. The C-reactive protein level after total knee arthroplasty is gender specific. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3163–3167. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, N.; Zhang, X.; Ji, W. C-reactive protein levels after 4 types of arthroplasty. Acta Orthop. 2009, 80, 330–333. [Google Scholar] [CrossRef]
- Yombi, J.C.; Schwab, P.E.; Thienpont, E. Serum C-Reactive Protein Distribution in Minimally Invasive Total Knee Arthroplasty Do Not Differ with Distribution in Conventional Total Knee Arthroplasty. PLoS ONE 2015, 10, e0124788. [Google Scholar] [CrossRef]
- Zavala, J.A.; Clark, J.C.; Kissenberth, M.J.; Tolan, S.J.; Hawkins, R.J. Management of deep infection after reverse total shoulder arthroplasty: A case series. J. Shoulder Elb. Surg. 2012, 21, 1310–1315. [Google Scholar] [CrossRef]
- Topolski, M.S.; Chin, P.Y.K.; Sperling, J.W.; Cofield, R.H. Revision shoulder arthroplasty with positive intraoperative cultures: The value of preoperative studies and intraoperative histology. J. Shoulder Elb. Surg. 2006, 15, 402–406. [Google Scholar] [CrossRef]
- Akgün, D.; Müller, M.; Perka, C.; Winkler, T. The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Jt. J. 2018, 100, 1482–1486. [Google Scholar] [CrossRef]


| Patient Overview | Total | Male | Female |
|---|---|---|---|
| Patients (n) | 280 | 114 | 166 |
| Age (mean y) (range) | 66 (28.1–88.5) | 61.2 (28.1–84.1) | 69.2 (30.2–88.5) |
| Hospital stay (mean d) (range) | 6.8 (2–14) | 6.5 (4–12) | 6.9 (2–14) |
| Hight (mean cm) (range) | 169 (148–194) | 177 (160–194) | 164 (148–180) |
| Weight (mean kg) (range) | 82.6 (40–139) | 89.7 (59–130) | 77.8 (40–139) |
| BMI | |||
| (mean kg/m2) (range) | 28.2 (16–46) | 28.2 (19–40) | 28.2 (16–46) |
| <30 | 176 (63%) | 71 (62.3%) | 105 (63.3%) |
| >30 | 104 (37%) | 43 (37.7%) | 61 (36.7%) |
| Side | |||
| Right | 155 (55%) | 63 (55.3%) | 92 (55.4%) |
| Left | 125 (45%) | 51 (44.7) | 74 (44.6) |
| Diagnosis | |||
| Primary omarthritis | 112 (40%) | 48 (42.1%) | 64 (38.6%) |
| Cuff tear arthropathy | 99 (35.4%) | 35 (30.7%) | 64 (38.6%) |
| Humeral head necrosis | 33 (11.8%) | 15 (13.1%) | 18 (10.8%) |
| Secondary omarthritis | 36 (12.9) | 16 (14%) | 20 (12%) |
| Procedure | |||
| Hemicap | 2 (0.7%) | 2 (1.8%) | 0 |
| Stemless | 33 (11.8) | 14 (12.3%) | 19 (11.4%) |
| TSA | 52 (18.6%) | 22 (19.3%) | 30 (18.1%) |
| RSA | 135 (48.2%) | 45 (39.5%) | 88 (53.0%) |
| HA with stem | 29 (10.4%) | 12 (10.5%) | 17 (10.2%) |
| Stemless HA | 29 (10.4%) | 19 (16.7%) | 10 (6%) |
| Operating time (min) (range) | 102.3 (48–380) | 106.5 (60–380) | 99.5 (48–186) |
| Cemented stem | |||
| Yes | 52 (18.6%) | 15 (13.2%) | 37 (22.3%) |
| No | 228 (81.4%) | 99 (86.8%) | 129 (77.7%) |
| Patients | Preoperative | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|---|
| Total group (n = 208) | <0.5 | 3.1 (1.9–4.2) | 8.9 (5–11.5) | 9.6 (5.3–13.1) | 7.3 (3.9–9.7) | 5 (2.6–9.8) | 4.3 (1.9–5.4) | 3.7 (2.0–4.6) |
| Male (n = 114) | <0.5 | 3 (1.9–3.8) | 9.2 (6.2–11.7) | 10 (5.7–13.4) | 7.8 (3.8–10.8) | 5.2 (2.6–7) | 5.4 (2.2–6.9) | 3.9 (2.2–4.7) |
| Female (n = 166) | <0.5 | 3.2 (1.9–4.4) | 8.5 (4.9–11.2) | 9.3 (5.1–13.1) | 6.8 (4.1–8.5) | 4.8 (2.6–6) | 3.6 (1.5–4.7) | 3.5 (1.7–4.9) |
| Patients | Preop | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| Total group (n = 280) | <0.5 | 3.1 | 8.9 | 9.6 | 7.3 | 5.0 | 4.3 | 3.7 |
| Male (n= 114) | <0.5 | 3.0 | 9.2 | 10.0 | 7.8 | 5.2 | 5.4 | 3.9 |
| Female (n= 166) | <0.5 | 3.2 | 8.5 | 9.3 | 6.8 | 4.8 | 3.6 | 3.5 |
| Anatomic prostheses | <0.5 | 2.6 | 8.0 | 7.4 | 6.6 | 4.0 | 3.6 | 3.1 |
| Hemicap (n = 2) | <0.5 | - | 1.3 | - | 0.9 | 0.5 | 0.5 | - |
| HA with stem (n = 29) | <0.5 | 1.8 | 9.0 | 8.6 | 5.8 | 3.4 | 2.9 | 0.6 |
| Stemless HA (n = 29) | <0.5 | 2.8 | 6.9 | 6.4 | 5.3 | 5.2 | 3.9 | 2.4 |
| Stemless TSA (n = 33) | <0.5 | 1.5 | 8.2 | 5.2 | 5.8 | 3.5 | 3.2 | 4.5 |
| TSA (n = 52) | <0.5 | 3.0 | 8.7 | 8.1 | 8.1 | 3.7 | 3.8 | 3.5 |
| RSA (n = 135) | <0.5 | 3.6 | 9.4 | 12.0 | 8.1 | 5.6 | 5.5 | 4.1 |
| BMI > 30 (n = 104) | <0.5 | 3.2 | 8.0 | 9.1 | 8.5 | 4.7 | 4.6 | 4.4 |
| BMI < 30 (n = 176) | <0.5 | 3.0 | 9.4 | 9.8 | 6.6 | 5.2 | 4.2 | 3.0 |
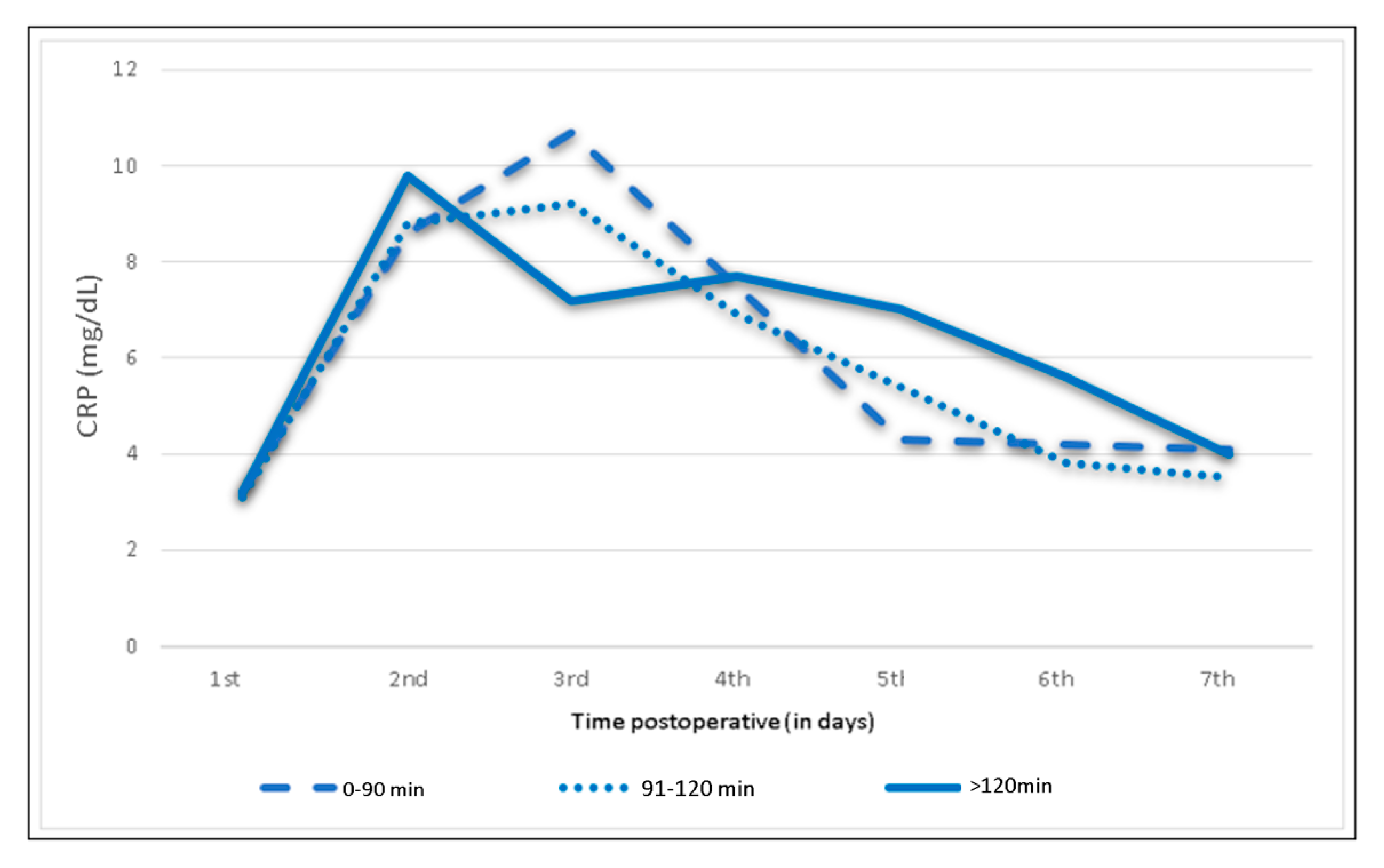
| OP time 0–90 min (n = 108) | <0.5 | 3.2 | 8.6 | 10.7 | 7.5 | 4.3 | 4.2 | 4.1 |
| 91–120 min (n = 125) | <0.5 | 3.1 | 8.8 | 9.2 | 6.9 | 5.4 | 3.8 | 3.5 |
| >120 min (n = 47) | <0.5 | 3.2 | 9.8 | 7.2 | 7.7 | 7.0 | 5.6 | 4.0 |
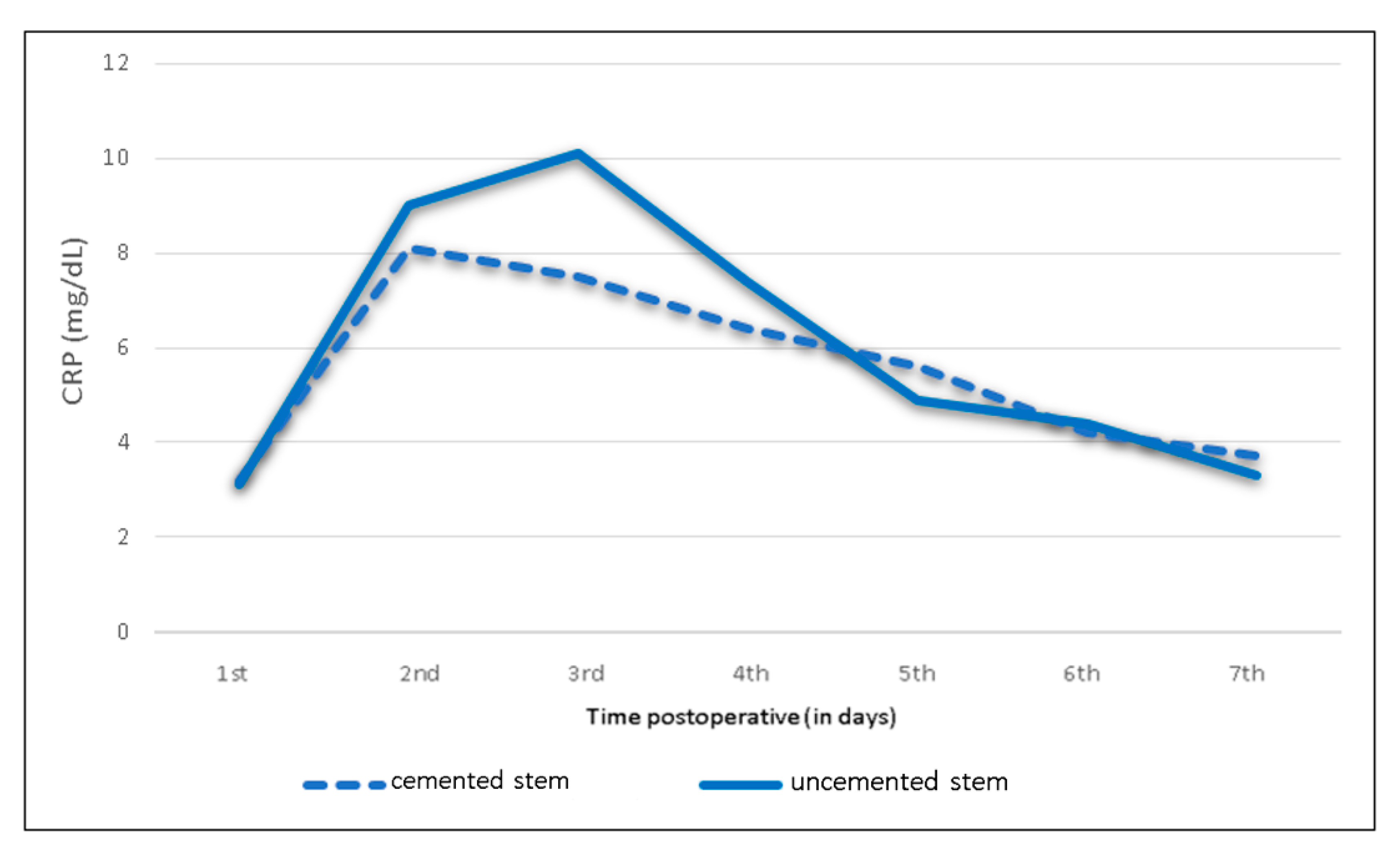
| Cemented stem (n= 52) | <0.5 | 3.2 | 8.1 | 7.5 | 6.4 | 5.5 | 4.2 | 3.7 |
| Uncemented stem (n= 228) | <0.5 | 3.1 | 9.0 | 10.1 | 7.4 | 4.9 | 4.4 | 3.3 |
| Patients | Preop | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| Total group (n = 280) | <0.5 | 3.1 | 8.9 | 9.6 | 7.3 | 5.0 | 4.3 | 3.7 |
| Male (n = 114) | <0.5 | 3.0 | 9.2 | 10.0 | 7.8 | 5.2 | 5.4 | 3.9 |
| Female (n = 166) | <0.5 | 3.2 | 8.5 | 9.3 | 6.8 | 4.8 | 3.6 | 3.5 |
| Anatomic prostheses | <0.5 | 2.6 | 8.0 | 7.4 | 6.6 | 4.0 | 3.6 | 3.1 |
| Hemicap (n = 2) | <0.5 | - | 1.3 | - | 0.9 | 0.5 | 0.5 | - |
| HA with stem (n = 29) | <0.5 | 1.8 | 9.0 | 8.6 | 5.8 | 3.4 | 2.9 | 0.6 |
| Stemless HA (n = 29) | <0.5 | 2.8 | 6.9 | 6.4 | 5.3 | 5.2 | 3.9 | 2.4 |
| Stemless TSA (n = 33) | <0.5 | 1.5 | 8.2 | 5.2 | 5.8 | 3.5 | 3.2 | 4.5 |
| TSA (n = 52) | <0.5 | 3.0 | 8.7 | 8.1 | 8.1 | 3.7 | 3.8 | 3.5 |
| RSA (n = 135) | <0.5 | 3.6 | 9.4 | 12.0 | 8.1 | 5.6 | 5.5 | 4.1 |
| BMI > 30 (n = 104) | <0.5 | 3.2 | 8.0 | 9.1 | 8.5 | 4.7 | 4.6 | 4.4 |
| BMI < 30 (n = 176) | <0.5 | 3.0 | 9.4 | 9.8 | 6.6 | 5.2 | 4.2 | 3.0 |
| OP time 0–90 min (n = 108) | <0.5 | 3.2 | 8.6 | 10.7 | 7.5 | 4.3 | 4.2 | 4.1 |
| 91–120 min (n = 125) | <0.5 | 3.1 | 8.8 | 9.2 | 6.9 | 5.4 | 3.8 | 3.5 |
| >120 min (n = 47) | <0.5 | 3.2 | 9.8 | 7.2 | 7.7 | 7.0 | 5.6 | 4.0 |
| Cemented stem (n = 52) | <0.5 | 3.2 | 8.1 | 7.5 | 6.4 | 5.5 | 4.2 | 3.7 |
| Uncemented stem (n = 228) | <0.5 | 3.1 | 9.0 | 10.1 | 7.4 | 4.9 | 4.4 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingebiel, S.; Theil, J.C.; Gosheger, G.; Schneider, K.N.; Timme, M.; Schorn, D.; Liem, D.; Rickert, C. Postoperative Trends of Serum C-Reactive Protein Levels after Primary Shoulder Arthroplasty—Normal Trajectory and Influencing Factors. J. Clin. Med. 2020, 9, 3893. https://doi.org/10.3390/jcm9123893
Klingebiel S, Theil JC, Gosheger G, Schneider KN, Timme M, Schorn D, Liem D, Rickert C. Postoperative Trends of Serum C-Reactive Protein Levels after Primary Shoulder Arthroplasty—Normal Trajectory and Influencing Factors. Journal of Clinical Medicine. 2020; 9(12):3893. https://doi.org/10.3390/jcm9123893
Chicago/Turabian StyleKlingebiel, Sebastian, Jan Christoph Theil, Georg Gosheger, Kristian Nikolaus Schneider, Maximilian Timme, Dominik Schorn, Dennis Liem, and Carolin Rickert. 2020. "Postoperative Trends of Serum C-Reactive Protein Levels after Primary Shoulder Arthroplasty—Normal Trajectory and Influencing Factors" Journal of Clinical Medicine 9, no. 12: 3893. https://doi.org/10.3390/jcm9123893
APA StyleKlingebiel, S., Theil, J. C., Gosheger, G., Schneider, K. N., Timme, M., Schorn, D., Liem, D., & Rickert, C. (2020). Postoperative Trends of Serum C-Reactive Protein Levels after Primary Shoulder Arthroplasty—Normal Trajectory and Influencing Factors. Journal of Clinical Medicine, 9(12), 3893. https://doi.org/10.3390/jcm9123893





