Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review
Abstract
:1. Introduction
1.1. The Problem in Dentistry
1.2. Oral Microbiota and Periodontal Disease
2. Materials and Methods
2.1. Focus Question
2.2. Elegibility Criteria
2.3. Search Strategy
2.4. Research
2.5. Screening and Selection of Articles
2.6. Search Outcome and Evaluation
3. Results
3.1. Study Selection
3.2. Characteristics of Studies
3.2.1. NSPT and Laser
3.2.2. NSPT and Ozone
3.2.3. NSPT and Airpolishing
3.2.4. NSPT and Probiotics
3.2.5. NSPT and Chlorhexidine
3.3. Synthesis of Results
3.3.1. PPD
PPD and Laser
PPD and Ozone
PPD and Airpolishing
PPD and Probiotics
PPD and Chlorhexidine
3.3.2. CAL
CAL and Laser
CAL and Ozone
CAL and Airpolishing
CAL and Probiotics
CAL and Chlorhexidine
3.3.3. BOP
BOP and Laser
BOP and Ozone
BOP and Airpolishing
BOP and Probiotics
BOP and Chlorhexidine
3.3.4. PI
PI and Laser
PI and Ozone
PI and Airpolishing
PI and Probiotics
PI and Chlorhexidine
3.3.5. Microbiological and Immunological Analysis
Microbiological and Immunological Analysis: Laser
Microbiological and Immunological Analysis: Ozone
Microbiological and Immunological Analysis: Airpolishing
Microbiological and Immunological Analysis: Probiotics
Microbiological and Immunological Analysis: Chlorhexidine
3.3.6. Results of Single Studies and Bias
4. Discussion
4.1. Rinse Pre-Treatment
4.2. Modified Full-Mouth Disinfection and Chlorhexidine
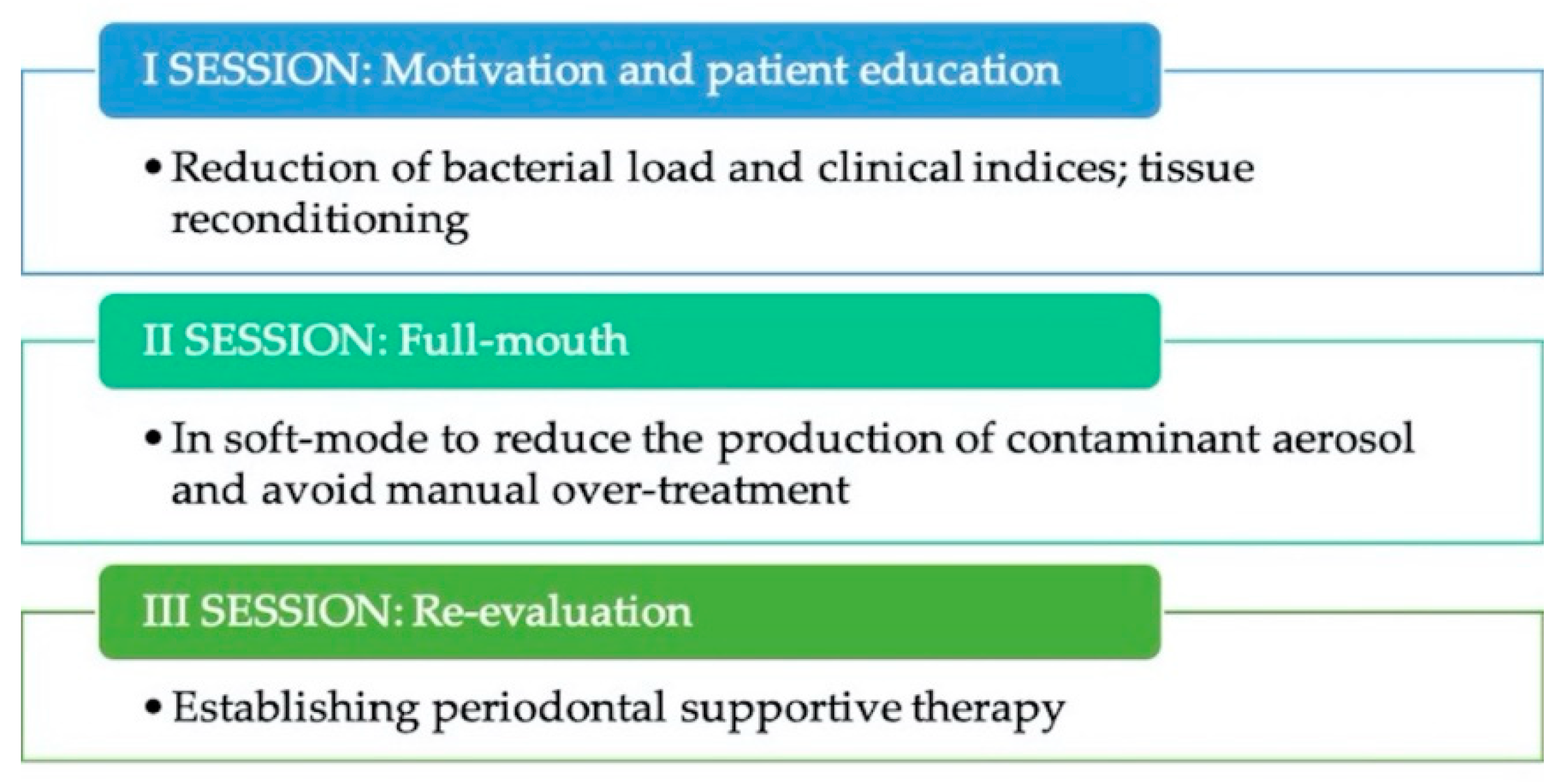
4.3. Measures for Modified Full-Mouth Disinfection
4.4. Laser Therapy
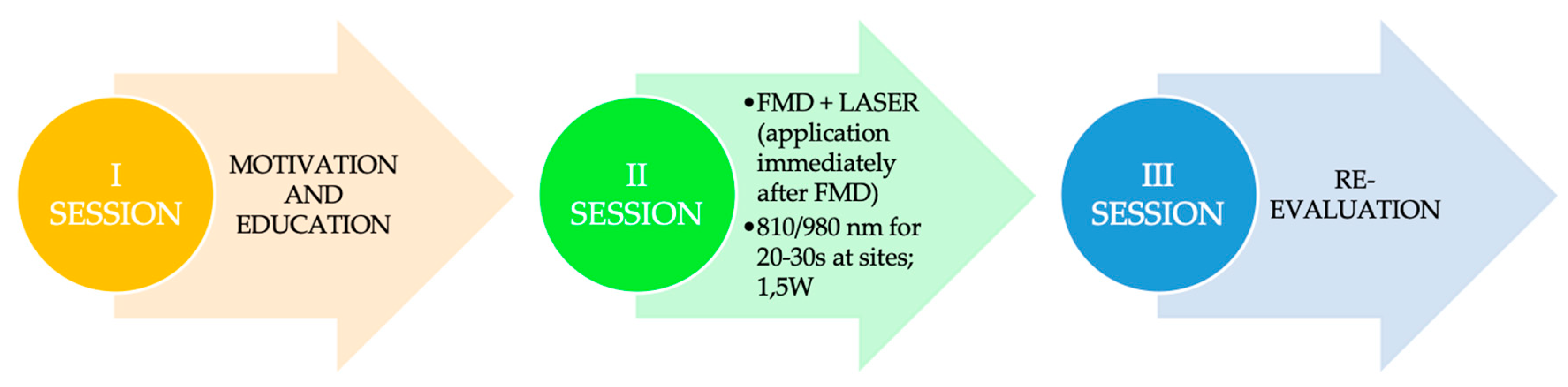
4.5. Measures for Laser Therapy in Modified Full-Mouth Disinfection
4.6. Ozonetherapy
4.7. Measures for Ozone Therapy in Modified Full-Mouth Disinfection
4.8. Airpolishing
4.9. Measures for Airpolishing
4.10. Home Hygiene Advice: The Probiotic Theme
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spagnuolo, G.; De Vito, D.; Rengo, S.; Tatullo, M. COVID-19 Outbreak: An overview on dentistry. Int. J. Environ. Res. Public Health 2020, 17, 2094. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/coronavirus/faq (accessed on 6 April 2020).
- Coulthard, P. Dentistry and coronavirus (COVID-19)—Moral decision-making. Br. Dent. J. 2020, 228, 503–505. [Google Scholar] [CrossRef]
- Available online: https://www.nytimes.com/interactive/2020/03/15/business/economy/coronavirus-worker-risk.html (accessed on 22 May 2020).
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef] [Green Version]
- French Society of Stomatology, Maxillo-Facial Surgery and Oral Surgery (SFSCMFCO). Practitioners specialized in oral health and coronavirus disease 2019: Professional guidelines from the French society of stomatology, maxillofacial surgery and oral surgery, to form a common front against the infectious risk. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 155–158. [Google Scholar] [CrossRef]
- Retamal-Valdes, B.; Soares, G.M.; Stewart, B.; Figueiredo, L.C.; Faveri, M.; Miller, S.; Zhang, Y.P.; Feres, M. Effectiveness of a pre-procedural mouthwash in reducing bacteria in dental aerosols: Randomized clinical trial. Braz. Oral Res. 2017, 31, e21. [Google Scholar] [CrossRef] [Green Version]
- Arabaci, T.; Ciçek, Y.; Canakçi, C.F. Sonic and ultrasonic scalers in periodontal treatment: A review. Int. J. Dent. Hyg. 2007, 5, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Auplisti, G.; Needleman, I.G.; Moles, D.R.; Newman, H.N. Diamond-coated sonic tips are more efficient for open debridment of molar furcations. A comparative manikin study. J. Clin. Periodontol. 2000, 27, 302–307. [Google Scholar] [CrossRef]
- Goldman, H.S.; Hartman, K.S.; Messite, J. Occupational Hazards in Dentistry; Year Book Medical Publishers: Chicago, IL, USA, 1984. [Google Scholar]
- Rivera-Hidalgo, F.; Barnes, J.B.; Harrel, S.K. Aerosol and splatter production by focused spray standard ultrasonic inserts. J. Periodontol. 1999, 70, 473–477. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High exspression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Bizzocca, M.E.; Campisi, G.; Lo Muzio, L. An innovative risk-scoring system of dental procedures and safety protocols in the COVID-19 era. BMC Oral Health 2020, 20, 301. [Google Scholar] [CrossRef]
- Trenter, S.C.; Walmsley, A.D. Ultrasonic dental scaler associated hazards. J. Clin. Periodontol. 2003, 30, 95–101. [Google Scholar] [CrossRef]
- Wirthlin, M.R.; Marshall, G.V. Evaluation of ultrasonic scaling unit waterline contamination after use of chlorine dioxide mouthrinse lavage. J. Periodontol. 2001, 72, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Yip, J.; Furgang, D.; Barnett, M.L.; Olshan, A.M.; Vincent, J. Reducing bacteria in dental aerosols: Pre-procedural use of an antiseptic mouthrinse. JADA 1993, 129, 1241–1249. [Google Scholar] [CrossRef]
- Available online: https://microbioma.it (accessed on 6 April 2020).
- Christgau, M.; Manner, T.; Beur, S.; Hiller, K.A.; Schmalz, G. Periodontal healing after non-surgical therapy with a new ultrasonic device: A randomized controlled clinical trial. J. Clin. Periodontol. 2002, 34, 137–147. [Google Scholar] [CrossRef]
- Cobbo, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. S2), 6–16. [Google Scholar] [CrossRef]
- Quirynen, M.; De Soete, M.; Dierickx, K.; Van Steenberghe, D. The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. J. Clin. Periodontol. 2001, 28, 499–507. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, M.; Zhang, D.; Song, Y.; Wang, Z. Efficacy of Er:YAG laser on periodontitis as an adjunctive non-surgical treatment: A split-mouth randomized controlled study. J. Clin. Periodontol. 2019, 46, 539–547. [Google Scholar] [CrossRef]
- Yilmaz, S.; Algan, S.; Gursoy, H.; Noyan, U.; Kuru, B.E.; Kadir, T. Evaluation of the clinical and antimicrobial effects of the Er:YAG laser or topical gaseous ozone as adjuncts to initial periodontal therapy. Photomed. Laser Surg. 2013, 31, 293–298. [Google Scholar] [CrossRef]
- Sağlam, M.; Köseoğlu, S.; Taşdemir, I.; Erbak Yılmaz, H.; Savran, L.; Sütçü, R. Combined application of Er:YAG and Nd:YAG lasers in treatment of chronic periodontitis. A split-mouth, single-blind, randomized controlled trial. J. Periodontal. Res. 2017, 52, 853–862. [Google Scholar] [CrossRef]
- Abduljabbar, T.; Vohra, F.; Kellesarian, S.V.; Javed, F. Efficacy of scaling and root planning with and without adjunct Nd:YAG laser therapy on clinical periodontal parameters and gingival crevicular fluid interleukin 1-beta and tumor necrosis factor-alpha levels among patients with periodontal disease: A prospective randomized split-mouth clinical study. J. Photochem. Photobiol. B 2017, 169, 70–74. [Google Scholar]
- Gou, H.; Fan, R.; Chen, X.; Li, L.; Wang, X.Q.; Xu, Y.; Svensson, P.; Wang, K.L. Adjunctive effects of laser therapy on somatosensory function and vasomotor regulation of periodontal tissues in patients with periodontitis- a randomized controlled clinical trial. J. Periodontol. 2020, 91, 1307–1317. [Google Scholar] [CrossRef]
- Talebi, J.M.; Taliee, R.; Mojahedi, M.; Meymandi, M.; Torshabi, M. Microbiological efficacy of photodynamic therapy as an adjunct to non-surgical periodontal treatment: A clinical trial. Lasers Med. Sci. 2016, 7, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir, Z.; Oskaybas, M.N.; Alkan, A.B.; Cakmak, O. The effects of ozone therapy on periodontal therapy: A randomized placebo-controlled clinical trial. Oral Dis. 2019, 25, 1195–1202. [Google Scholar] [CrossRef]
- Hayakumo, S.; Arakawa, S.; Mano, Y.; Izumi, Y. Clinical and microbiological effects of ozone nano-bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. Clin. Oral Investig. 2013, 17, 379–388. [Google Scholar] [CrossRef]
- Kshitish, D.; Laxman, V.K. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: A clinical and microbiologic study. Indian J. Dent. Res. 2010, 21, 341–348. [Google Scholar]
- Lu, H.; He, L.; Zhao, Y.; Meng, H. The effect of supragingival glycine air polishing on periodontitis during maintenance therapy: A randomized controlled trial. PeerJ 2018, 6, e437. [Google Scholar] [CrossRef] [Green Version]
- Wennström, J.L.; Dahlén, G. Ramberg, Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J. Clin. Periodontol. 2011, 38, 820–827. [Google Scholar] [CrossRef]
- Müller, N.; Moëne, R.; Cancela, J.A.; Mombelli, A. Subgingival air-polishing with erythritol during periodontal maintenance: Randomized clinical trial of twelve months. J. Clin. Periodontol. 2014, 41, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Pelekos, G.; Ho, S.N.; Acharya, A.; Leung, W.K.; McGrath, C. A double-blind, paralleled-arm, placebo-controlled and randomized clinical trial of the effectiveness of probiotics as an adjunct in periodontal care. J. Clin. Periodontol. 2019, 46, 1217–1227. [Google Scholar] [CrossRef]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [Green Version]
- De Costa, F.N.L.; Amaral, C.D.S.F.; Barbirato, D.D.S.; Leão, A.T.T.; Fogacci, M.F. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J. Am. Dent. Assoc. 2017, 148, 308–318. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. Adjunctive subgingival application of chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 34. [Google Scholar] [CrossRef] [Green Version]
- Paolantonio, M.; D’Angelo, M.; Grassi, R.F.; Perinetti, G.; Piccolomini, R.; Pizzo, G.; Annunziata, M.; D’Archivio, D.; D’Ercole, S.; Nardi, G.; et al. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: A multicenter study. J. Clin. Periodontol. 2008, 79, 271–282. [Google Scholar] [CrossRef]
- Manthena, S.; Ramesh, A.; Srikanth, A.; Rao, R.M.V.; Preethi, L.P.; Pallavi, S.Y. Comparative evaluation of subgingivally delivered chlorhexidine varnish and chlorhexidine gel in reducing microbial count after mechanical periodontal therapy. J. Basic Clin. Pharm. 2014, 6, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef] [Green Version]
- Devker, N.R.; Mohitey, J.; Vibhute, A.; Chouhan, V.S.; Chavan, P.; Malagi, S.; Joseph, R. A study to evaluate and compare the efficacy of preprocedural mouthrinsing and high volume evacuator attachment alone and in combination in reducing the amount of viable aerosols produced during ultrasonic scaling procedure. J. Contemp. Dent. Pract. 2012, 13, 681–689. [Google Scholar]
- Tomás, I.; Alvarez, M.; Limeres, J.; Tomás, M.; Medina, J.; Otero, J.L.; Diz, P. Effect of a chlorhexidine mouthwash on the risk of postextraction bacteremia. Infect. Control. Hosp. Epidemiol. 2007, 28, 577–582. [Google Scholar] [CrossRef]
- Horliana, A.C.; Chambrone, L.; Foz, A.M.; Artese, H.P.C.; de Sousa Rabelo, M.; Pannuti, C.M.; Romito, G.A. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: A systematic review. PLoS ONE 2014, 9, e98271. [Google Scholar] [CrossRef] [Green Version]
- Kinane, D.F.; Riggio, M.P.; Walker, K.F.; MacKenzie, D.; Shearer, B. Bacteraemia following periodontal procedures. J. Clin. Periodontol. 2005, 32, 708–713. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Debiaggi, M.; Zara, F.; Brerra, R.; Comelli, M.; Bianchi, M.; Pollone, S.R.; Scribante, A. Influence of lingual bracket position on microbial and periodontal parameters in vivo. J. Appl. Oral Sci. 2012, 20, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [Green Version]
- Scribante, A.; Sfondrini, M.F.; Collesano, V.; Tovt, G.; Bernardinelli, L.; Gandini, P. Dental Hygiene and Orthodontics: Effect of Ultrasonic Instrumentation on Bonding Efficacy of Different Lingual Orthodontic Brackets. BioMed Res. Int. 2017, 2017, 3714651. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Nishimura, F.; Kurihara, M.; Ywamoto, Y.; Takashiba, S.; Miyata, T.; Murayama, Y. Subgingival microflora and antibody responses against periodontal bacteria of young Japanese patients with type 1 diabetes mellitus. J. Int. Acad. Periodontol. 2001, 3, 104–111. [Google Scholar]
- Paizan, M.L.M.; Vilela-Martin, J.F. Is there an Association between Periodontitis and Hypertension? Curr. Cardiol. Rev. 2014, 10, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Presha, P.M.; Alba, A.L. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Aguiler, E.M.; Suva, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef]
- Dukić, W.; Bago, I.; Aurer, A.; Roguljić, M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized clinical study. J. Periodontol. 2013, 84, 1111–1117. [Google Scholar] [CrossRef]
- De Melo Soares, M.S.; D’Almeida Borges, C.; de Mendonça Invernici, M.; Frantz, F.G.; de Figueiredo, L.C.; de Souza, S.L.S.; Taba, M., Jr.; Messora, M.R.; Novaes, A.B., Jr. Antimicrobial photodynamic therapy as adjunct to non-surgical periodontal treatment in smokers: A randomized clinical trial. Clin. Oral Investig. 2019, 23, 3173–3182. [Google Scholar] [CrossRef]
- Zengin Celik, T.; Saglam, E.; Ercan, C.; Akbas, F.; Nazaroglu, K.; Tunali, M. Clinical and microbiological effects of the se of erbium: Yttrium-aluminum-garnet laser on chronic periodontitis in addition to nonsurgical periodontal treatment: A randomized clinical trial-6 months follow-up. Photomed. Laser Surg. 2019, 37, 182–190. [Google Scholar] [CrossRef]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Matos, R.; Herrera, D.; Sanz, M. Clinical efficacy of subgingival debridement with adjunctive erbium:yttrium-aluminum-garnet lasertreatment in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2015, 86, 527–535. [Google Scholar] [CrossRef]
- Gündoğar, H.; Şenyurt, S.Z.; Erciyas, K.; Yalım, M.; Üstün, K. The effect of low-level laser therapy on non-surgical periodontal treatment: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2016, 31, 1767–1773. [Google Scholar] [CrossRef]
- Üstün, K.; Erciyas, K.; Sezer, U.; Şenyurt, S.Z.; Gündoğar, H.; Üstün, Ö.; Öztuzcu, S. Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: A randomized split-mouth clinical trial. Photomed. Laser Surg. 2014, 32, 61–66. [Google Scholar] [CrossRef]
- Dereci, Ö.; Hatipoğlu, M.; Sindel, A.; Tozoğlu, S.; Üstün, K. The efficacy of Er,Cr:YSGG laser supported periodontal therapy on the reduction of peridodontal disease related oral malodor: A randomized clinical study. Head Face Med. 2016, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Bundidpun, P.; Srisuwantha, R.; Laosrisin, N. Clinical effects of photodynamic therapy as an adjunct to full-mouth ultrasonic scaling and root planing in treatment of chronic periodontitis. Laser Ther. 2018, 27, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Jose, K.A.; Ambooken, M.; Mathew, J.J.; Issac, A.V.; Kunju, A.P.; Parameshwaran, R.A. Management of chronic periodontitis using chlorhexidine chip and diode laser-a clinical study. J. Clin. Diagn. Res. 2016, 10, ZC76–ZC80. [Google Scholar] [CrossRef]
- Morales, A.; Carvajal, P.; Silva, N.; Hernandez, M.; Godoy, C.; Rodriguez, G.; Cabello, R.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.I.; et al. Clinical effects of lactobacillus rhamnosus in non-surgical treatment of chronic periodontitis: A randomized placebo-controlled trial with 1-year follow-up. J. Periodontol. 2016, 87, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Penala, S.; Kalakonda, B.; Pathakota, K.R.; Jayakumar, A.; Koppolu, P.; Lakshmi, B.V.; Pandey, R.; Mishra, A. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: A randomized controlled trial. J. Res. Pharm. Pract. 2016, 5, 86–93. [Google Scholar] [CrossRef]
- Lecic, J.; Cakic, S.; Janjic Pavlovic, O.; Cicmil, A.; Vukotic, O.; Petrovic, V.; Cicmil, S. Different methods for subgingival application of chlorhexidine in the treatment of patients with chronic periodontitis. Acta Odontol. Scand. 2016, 74, 502–507. [Google Scholar] [CrossRef]
- Lopes, B.M.; Theodoro, L.H.; Melo, R.F.; Thompson, G.M.; Marcantonio, R.A. Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with erbium:YAG laser and scaling and root planning. J. Periodontol. 2010, 81, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Daghig Azar, B.; Farhadi, F. Clinical and microbiological effects of photodynamic therapy associated with non-surgical treatment in aggressive periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 153–159. [Google Scholar]
- Grzech-Leśniak, K.; Sculean, A.; Gašpirc, B. Laser reduction of specific microorganisms in the periodontal pocket using Er:YAG and Nd:YAG lasers: A randomized controlled clinical study. Lasers Med. Sci. 2018, 33, 1461–1470. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Cicciù, M.; Cordasco, G.; Isola, G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-year randomized controlled clinical trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef]
- Seydanur Dengizek, E.; Serkan, D.; Abubekir, E.; Bay, K.; Otlu, O.; Arife, C. Evaluating clinical and laboratory effects of ozone in non-surgical periodontal treatment: A randomized controlled trial. J. Appl. Oral Sci. 2019, 27, e20180108. [Google Scholar] [CrossRef]
- Uraz, A.; Karaduman, B.; Isler, S.Ç.; Gönen, S.; Çetiner, D. Ozone application as adjunctive therapy in chronic periodontitis: Clinical, microbiological and biochemical aspects. J. Dent. Sci. 2019, 14, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Skurska, A.; Pietruska, M.D.; Paniczko-Drężek, A.; Dolińska, E.; Zelazowska-Rutkowska, B.; Zak, J.; Pietruski, J.; Milewski, R.; Wysocka, J. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv. Med. Sci. 2010, 55, 297–307. [Google Scholar] [CrossRef]
- Park, E.J.; Kwon, E.Y.; Kim, H.J.; Lee, J.Y.; Choi, J.; Joo, J.Y. Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: A randomized clinical trial. J. Periodontal. Implant. Sci. 2018, 48, 295–304. [Google Scholar] [CrossRef]
- Tsang, Y.C.; Corbet, E.F.; Jin, L.J. Subgingival glycine powder air-polishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J. Periodontal. Res. 2018, 53, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Yılmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef]
- Vivekananda, M.R.; Vandana, K.L.; Bhat, K.G. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. J. Oral Microbiol. 2010, 2, 5344. [Google Scholar] [CrossRef]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and biochemical evaluation of lozenges containing lactobacillus reuteri as an adjunct to non-surgical periodontal therapy in chronic periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef]
- John, P.; Lazarus, F.; George, J.P.; Selvam, A.; Prabhuji, M.L. Adjunctive effects of a piscean collagen-based controlled-release chlorhexidine chip in the treatment of chronic periodontitis: A clinical and microbiological study. J. Clin. Diagn. Res. 2015, 9, ZC70–ZC74. [Google Scholar] [CrossRef]
- Gonzales, J.R.; Harnack, L.; Schmitt-Corsitto, G.; Boedeker, R.H.; Chakraborty, T.; Domann, E.; Meyle, J. A novel approach to the use of subgingival controlled-release chlorhexidine delivery in chronic periodontitis: A randomized clinical trial. J. Periodontol. 2011, 82, 1131–1139. [Google Scholar] [CrossRef]
- García-Gargallo, M.; Zurlohe, M.; Montero, E.; Alonso, B.; Serrano, J.; Sanz, M.; Herrera, D. Evaluation of new chlorhexidine- and cetylpyridinium chloride-based mouthrinse formulations adjunctive to scaling and root planing: Pilot study. Int. J. Dent. Hyg. 2017, 15, 269–279. [Google Scholar] [CrossRef]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015, 60, 1247–1256. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, K.P.; Kim, H.K.; Kim, S.G.; Kook, J.K.; Park, S.N. Salivary bacterial counts after application of povidone-iodine and chlorhexidine. J. Korean Assoc. Oral Maxillofac. Surg. 2009, 35, 312–510. [Google Scholar]
- Ricevuti, G.; Franzini, M.; Valdenassi, L. Oxygen-ozone immunoceutical therapy in COVID-19 outbreak: Facts and figures. Ozone Ther. 2020, 5, 9014. [Google Scholar] [CrossRef] [Green Version]
- Anand, V.; Govila, V.; Gulati, M.; Anand, B.; Jhingaran, R.; Rastogi, P. Chlorhexidine-thymol varnish as an adjunct to scaling and root planing: A clinical observation. J. Oral Biol. Craniofac. Res. 2012, 2, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Sridhar, R.; Shrihatti, R.; Mandloy, A. Evaluation of turmeric chip compared with chlorhexidine chip as a local drug delivery agent in the treatment of chronic periodontitis: A split mouth randomized controlled clinical trial. J. Altern. Complement. Med. 2018, 24, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Dave, D.; Jain, P.; Manohar, B.; Yadav, B.; Shetty, N. Efficacy of xanthan based chlorhexidine gel as an adjunct to scaling and root planing in treatment of the chronic periodontitis. J. Indian Soc. Periodontol. 2013, 17, 439–443. [Google Scholar] [CrossRef]
- Chitsazi, M.T.; Kashefimehr, A.; Pourabbas, R.; Shirmohammadi, A.; Ghasemi Barghi, V.; Daghigh Azar, B. Efficacy of subgingival application of xanthan-based chlorhexidine gel adjunctive to full-mouth root planing assessed by Real-time PCR: A microbiologic and clinical study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2013, 7, 95–101. [Google Scholar]
- Calderini, A.; Pantaleo, G.; Rossi, A.; Gazzolo, D.; Polizzi, E. Adjunctive effect of chlorhexidine antiseptics in mechanical periodontal treatment: First results of a preliminary case series. Int. J. Dent. Hyg. 2013, 11, 180–185. [Google Scholar] [CrossRef]
- Fonseca, D.C.; Cortelli, J.R.; Cortelli, S.C.; Miranda Cota, L.O.; Machado Costa, L.C.; Moreira Castro, M.V.; Oliveira Azevedo, A.M.; Costa, F.O. Clinical and microbiologic evaluation of scaling and root planing per quadrant and one-stage full-mouth disinfection associated with azithromycin or chlorhexidine: A clinical randomized controlled trial. J. Periodontol. 2015, 86, 1340–1351. [Google Scholar] [CrossRef]
- Medaiah, S.; Srinivas, M.; Melath, A.; Girish, S.; Polepalle, T.; Dasari, A.B. Chlorhexidine chip in the treatment of chronic periodontitis—A clinical study. J. Clin. Diagn. Res. 2014, 8, ZC22–ZC25. [Google Scholar]
- Krück, C.; Eick, S.; Knöfler, G.U.; Purschwitz, R.E.; Jentsch, H.F. Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: A pilot randomized trial. J. Periodontol. 2012, 83, 312–320. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Vandekerckhove, B.N.; Dekeyser, C.; Papaioannou, W.; Eyssen, H. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: Short-term clinical and microbiological observations. J. Dent. Res. 1995, 74, 1459–1467. [Google Scholar] [CrossRef]
- Genovesi, A.M.; Marconcini, S.; Ricci, M.; Marchisio, O.; Covani, F.; Covani, U. Evaluation of a decontamination protocol prior to a full-mouth disinfection procedure: A randomized clinical study. J. Dent. Oral Hyg. 2014, 6, 77–84. [Google Scholar]
- Marconcini, S.; Goulding, M.; Oldoini, G.; Attanasio, C.; Giammarinaro, E.; Genovesi, A. Clinical and patient-centered outcomes post non-surgical periodontal therapy with the use of a non-injectable anesthetic product: A randomized clinical study. J. Investig. Clin. Dent. 2019, 10, e12446. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Aoki, A.; Becker, J.; Sculean, A. Laser application in non-surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 29–44. [Google Scholar] [CrossRef]
- Manjunath, S.; Singla, D.; Singh, R. Clinical and microbiological evaluation of the synergistic effects of diode laser with nonsurgical periodontal therapy: A randomized clinical trial. J. Indian Soc. Periodontol. 2020, 24, 145–149. [Google Scholar]
- Euzebio Alves, V.T.; de Andrade, A.K.; Toaliar, J.M.; Conde, M.C.; Zezell, D.M.; Cai, S.; Pannuti, C.M.; De Micheli, G. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin. Oral Investig. 2013, 17, 87–95. [Google Scholar] [CrossRef]
- Berakdar, M.; Callaway, A.; Eddin, M.F.; Ross, A.; Willershausen, B. Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: Six-month study. Head Face Med. 2012, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Shergill, N. The effect of low-level laser therapy on nonsurgical periodontal therapy: A clinico-biochemical study. J. Dent. Lasers 2018, 12, 14–17. [Google Scholar] [CrossRef]
- Aykol, G.; Baser, U.; Maden, I.; Kazak, Z.; Onan, U.; Tanrikulu-Kucuk, S.; Ademoglu, E.; Issever, H.; Yalcin, F. The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J. Periodontol. 2011, 82, 481–488. [Google Scholar] [CrossRef]
- Petrović, M.S.; Kannosh, I.Y.; Milašin, J.M. Clinical, microbiological and cytomorphometric evaluation of low-level laser therapy as an adjunct to periodontal therapy in patients with chronic periodontitis. J. Int. Dent. Hyg. 2018, 16, e120–e127. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Herrera, D.; Sanz, M. Microbiological effects and recolonization patterns after adjunctive subgingival debridement with Er:YAG laser. Clin. Oral Investig. 2016, 20, 1253–1261. [Google Scholar] [CrossRef]
- Üstun, K.; Hatipoglu, M.; Daltaban, O.; Felek, R.; Firat, M.Z. Clinical and biochemical effects of erbium, chromium: Yttrium, scandium, gallium, garnet laser treatment as a complement to periodontal treatment. Niger J. Clin. Pract. 2018, 21, 1150–1157. [Google Scholar]
- Gupta, G.; Mansi, B. Ozone therapy in periodontics. Med. J. Life 2012, 5, 59–67. [Google Scholar]
- Srikanth, A.; Sathish, M.; Sri Harsha, A.V. Application of ozone in the treatment of periodontal disease. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. S1), S89–S94. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Mollica, P.; Harris, R. Integrating Oxygen/Ozone Therapy into Your Practice. Available online: www.acimd.net (accessed on 13 January 2010).
- Gandhi, K.K.; Cappetta, E.G.; Pavaskar, R. Effectiveness of the adjunctive use of ozone and chlorhexidine in patients with chronic periodontitis. BDJ Open 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.; Patel, S.; Kaitlyn, R.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. Clinical utility of ozone therapy in dental and oral medicine. Med. Gas. Res. 2019, 9, 163–167. [Google Scholar]
- Bezrukova, I.V.; Petrukhina, N.B.; Voinov, P.A. Experience in medical ozone use for root canal treatment. Stomatologiia 2005, 84, 20–22. [Google Scholar]
- Petersilka, G.J.; Steinmann, D.; Häberlein, I.; Heinecke, A.; Flemmig, T.F. Subgingival plaque removal in buccal and lingual sites using a novel low abrasive air-polishing powder. J. Clin. Periodontol. 2003, 30, 328–333. [Google Scholar] [CrossRef]
- Farronato, M.; Boccalari, E.; Del Rosso, E.; Lanteri, V.; Mulder, R.; Maspero, C. A Scoping Review of Respirator Literature and a Survey among Dental Professionals. Int. J. Environ. Res. Public Health 2020, 17, 5968. [Google Scholar] [CrossRef]
- Cobb, C.M.; Daubert, D.M.; Davis, K. Consensus conference findings on supragingival and subgingival air polishing. Compend. Contin. Educ. Dent. 2017, 38, e1–e4. [Google Scholar]
- Ng, E.; Byun, R.; Spahr, A.; Divnic-Resnik, T. The efficacy of air polishing devices in supportive periodontal therapy: A systematic review and meta-analysis. Quintessence Int. 2018, 49, 453–467. [Google Scholar] [PubMed]
- Hägi, T.T.; Hofmänner, P.; Salvi, G.E.; Ramseier, C.A.; Sculean, A. Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive periodontal therapy: A randomized, controlled clinical study. Quintessence Int. 2013, 44, 753–761. [Google Scholar]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef]




| Adequate Sequence Generated | Allocation Concealment | Blinding | Incomplete Outcome Data | Registration Outcome | |
|---|---|---|---|---|---|
| Dukić 2013 |  |  |  |  |  |
| de Melo Soares 2019 |  |  |  |  |  |
| Moreira 2015 |  |  |  |  |  |
| Gündoğar 2016 |  |  |  |  |  |
| Hayakumo 2013 |  |  |  |  |  |
| Teughels 2013 |  |  |  |  |  |
| Lecic 2016 |  |  |  |  |  |
| Park 2018 |  |  |  |  |  |
| Vivekananda 2010 |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Maiorani, C.; Natoli, V.; Bruni, A.; Coscione, C.; Magliano, G.; Giacobbo, G.; Morelli, A.; Moressa, S.; Scribante, A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. J. Clin. Med. 2020, 9, 3914. https://doi.org/10.3390/jcm9123914
Butera A, Maiorani C, Natoli V, Bruni A, Coscione C, Magliano G, Giacobbo G, Morelli A, Moressa S, Scribante A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. Journal of Clinical Medicine. 2020; 9(12):3914. https://doi.org/10.3390/jcm9123914
Chicago/Turabian StyleButera, Andrea, Carolina Maiorani, Valentino Natoli, Ambra Bruni, Carmen Coscione, Gaia Magliano, Giulia Giacobbo, Alessia Morelli, Sara Moressa, and Andrea Scribante. 2020. "Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review" Journal of Clinical Medicine 9, no. 12: 3914. https://doi.org/10.3390/jcm9123914
APA StyleButera, A., Maiorani, C., Natoli, V., Bruni, A., Coscione, C., Magliano, G., Giacobbo, G., Morelli, A., Moressa, S., & Scribante, A. (2020). Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. Journal of Clinical Medicine, 9(12), 3914. https://doi.org/10.3390/jcm9123914








