The Risk of Preterm Birth in Women with Three Consecutive Deliveries—The Effect of Number and Type of Prior Preterm Births
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Exposure
2.3. Outcomes
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Characteristics of the Study Population
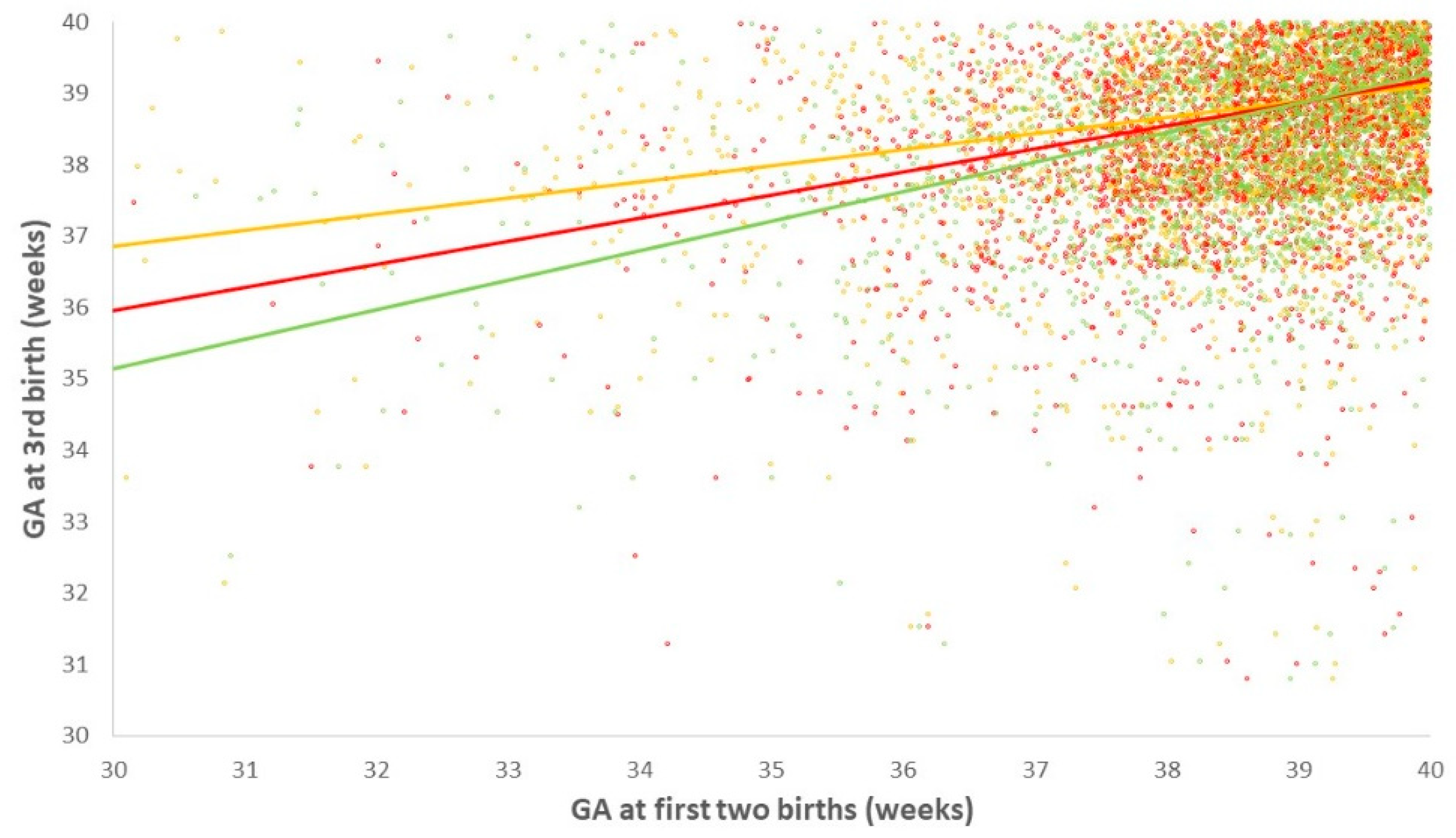
3.2. Number and Gestational Age at Prior PTB and the Risk for PTB in the Third Delivery
3.3. The Association of the Type of Prior PTB and the Risk for PTB in the Third Delivery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Jarjour, I.T. Neurodevelopmental Outcome after Extreme Prematurity: A Review of the Literature. Pediatr. Neurol. 2015, 52, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Natarajan, G. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.A.; Klebanoff, M.A. The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. 3), 17–42. [Google Scholar] [CrossRef]
- Melamed, N.; Hiersch, L.; Meizner, I.; Bardin, R.; Wiznitzer, A.; Yogev, Y. Is measurement of cervical length an accurate predictive tool in women with a history of preterm delivery who present with threatened preterm labor? Ultrasound Obstet. Gynecol. 2014, 44, 661–668. [Google Scholar] [CrossRef][Green Version]
- Mercer, B.M.; Goldenberg, R.L.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.F.; Caritis, S.N.; Miodovnik, M.; Menard, M.; Thurnau, G.R.; et al. The Preterm Prediction Study: Effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am. J. Obstet. Gynecol. 1999, 181, 1216–1221. [Google Scholar] [CrossRef]
- Schaaf, J.M.; Ravelli, A.C.; Mol, B.W.J.; Abu-Hanna, A. Development of a prognostic model for predicting spontaneous singleton preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 150–155. [Google Scholar] [CrossRef]
- Ananth, C.V.; Getahun, D.; Peltier, M.R.; Salihu, H.M.; Vintzileos, A.M. Recurrence of spontaneous versus medically indicated preterm birth. Am. J. Obstet. Gynecol. 2006, 195, 643–650. [Google Scholar] [CrossRef]
- Kazemier, B.M.; Buijs, P.; Mignini, L.; Limpens, J.; De Groot, C.; Mol, B.; Connect, E. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1197–1208. [Google Scholar] [CrossRef]
- Phillips, C.; Velji, Z.; Hanly, C.; Metcalfe, A. Risk of recurrent spontaneous preterm birth: A systematic review and meta-analysis. BMJ Open 2017, 7, e015402. [Google Scholar] [CrossRef]
- Yang, J.; Baer, R.J.; Berghella, V.; Chambers, C.; Chung, P.; Coker, T.; Currier, R.J.; Druzin, M.L.; Kuppermann, M.; Muglia, L.J.; et al. Recurrence of Preterm Birth and Early Term Birth. Obstet. Gynecol. 2016, 128, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Esplin, M.S.; O’Brien, E.; Fraser, A.; Kerber, R.A.; Clark, E.; Simonsen, S.; Holmgren, C.; Mineau, G.P.; Varner, M.W. Estimating Recurrence of Spontaneous Preterm Delivery. Obstet. Gynecol. 2008, 112, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, E.I.; Ravelli, A.C.J.; Koullali, B.; Kazemier, B.; De Groot, C.J.; Mol, B.W.J. Spontaneous and iatrogenic preterm birth rates among unselected women in three consecutive pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Laughon, S.K.; Albert, P.S.; Leishear, K.; Mendola, P. The NICHD Consecutive Pregnancies Study: Recurrent preterm delivery by subtype. Am. J. Obstet. Gynecol. 2014, 210, 131.e1–131.e8. [Google Scholar] [CrossRef]
- McManemy, J.; Cooke, E.; Amon, E.; Leet, T. Recurrence risk for preterm delivery. Am. J. Obstet. Gynecol. 2007, 196, 576.e1–576.e7. [Google Scholar] [CrossRef]
- Lucovnik, M.; Bregar, A.T.; Steblovnik, L.; Verdenik, I.; Gersak, K.; Blickstein, I.; Tul, N. Changes in incidence of iatrogenic and spontaneous preterm births over time: A population-based study. J. Périnat. Med. 2016, 44, 505–509. [Google Scholar] [CrossRef]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.-S. Birth weight standards in the live-born population in Israel. Israel Med. Assoc. J. 2005, 7, 311–344. [Google Scholar]
- Denney, J.M.; Waters, T.P.; Mathew, L.; Goldenberg, R.; Culhane, J. Variation in C-reactive protein at 1 month post-partum by etiology of preterm birth: Selective identification of those at risk for both poor pregnancy outcome and future health complications. J. Périnat. Med. 2019, 47, 804–810. [Google Scholar] [CrossRef]
- Bandoli, G.; Jelliffe-Pawlowski, L.L.; Feuer, S.K.; Liang, L.; Oltman, S.P.; Paynter, R.; Ross, K.M.; Schetter, C.D.; Ryckman, K.K.; Chambers, C.D. Second trimester serum cortisol and preterm birth: An analysis by timing and subtype. J. Perinatol. 2018, 38, 973–981. [Google Scholar] [CrossRef]
- Levine, L.D.; Bogner, H.R.; Hirshberg, A.; Elovitz, M.A.; Sammel, M.D.; Srinivas, S.K. Term induction of labor and subsequent preterm birth. Am. J. Obstet. Gynecol. 2014, 210, 354.e1–354.e8. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Lutsiv, O.; Beyene, J.; McDonald, S.D. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: An updated systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. The choice of progestogen for the prevention of preterm birth in women with singleton pregnancy and prior preterm birth. Am. J. Obstet. Gynecol. 2017, 216, B11–B13. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy; NICE: London, UK, 2010.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 743: Low-Dose Aspirin Use during Pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- Duley, L.; Henderson-Smart, D.J.; Meher, S.; King, J.F. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2007, 2, CD004659. [Google Scholar] [CrossRef]
- Hiersch, L.; Shinar, S.; Melamed, N.; Aviram, A.; Hadar, E.; Yogev, Y.; Ashwal, E. Recurrent Placenta-Mediated Complications in Women with Three Consecutive Deliveries. Obstet. Gynecol. 2017, 129, 416–421. [Google Scholar] [CrossRef]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J.; on behalf of the Knowledge Synthesis Group. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 2010, 341, c3428. [Google Scholar] [CrossRef]
- Premkumar, A.; Henry, D.E.; Moghadassi, M.; Nakagawa, S.; Norton, M.E. The interaction between maternal race/ethnicity and chronic hypertension on preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 787.e1–787.e8. [Google Scholar] [CrossRef]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef]
- Romero, R.; Yeo, L.; Chaemsaithong, P.; Chaiworapongsa, T.; Hassan, S.S. Progesterone to prevent spontaneous preterm birth. Semin. Fetal Neonatal Med. 2014, 19, 15–26. [Google Scholar] [CrossRef]
- Blackwell, S.C.; Chauhan, S.P.; Gyamfi-Bannerman, C.; Biggio, J.R.; Hughes, B.L.; Louis, J.M.; Manuck, T.A.; Miller, H.S.; Das, A.F.; Saade, G.R.; et al. 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double-Blind Trial. Am. J. Perinatol. 2020, 37, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Nicolaides, K.H. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 42–65.e2. [Google Scholar] [CrossRef] [PubMed]
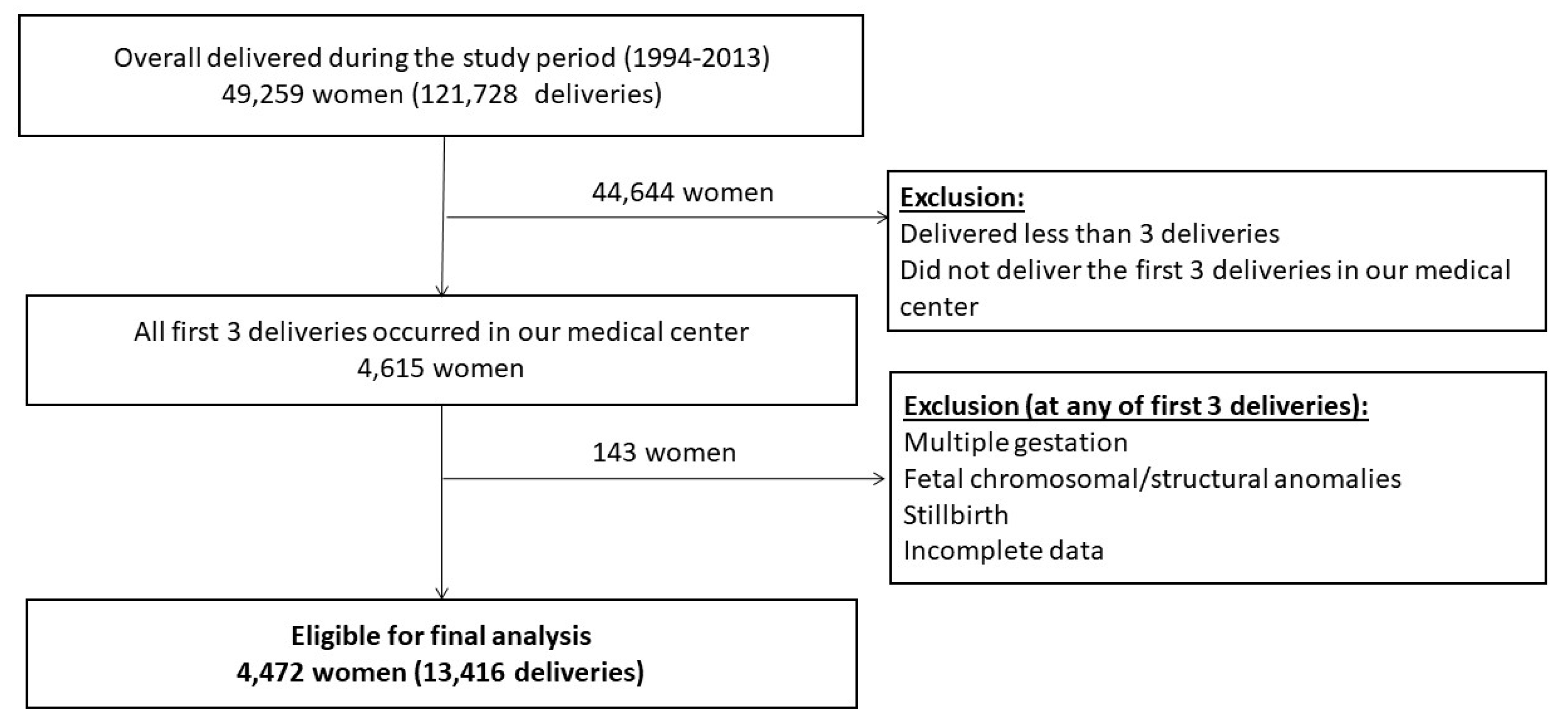

| Variable | 1st Delivery (n = 4472) | 2nd Delivery (n = 4472) | 3rd Delivery (n = 4472) |
|---|---|---|---|
| Maternal Age (y) * | 26.4 ± 3.8 | 29.1 ± 3.9 | 32.5 ± 4.2 |
| Maternal age > 35 y * | 74 (1.7) | 245 (5.5) | 1513 (33.8) |
| Hypertensive disorders * | 170 (3.8) | 56 (1.3) | 42 (0.9) |
| Gestational age at delivery (weeks) * | 39.4 ± 1.7 | 39.0 ± 1.6 | 38.9 ± 1.6 |
| PTB < 37 weeks * | 269 (6.0) | 214 (4.8) | 217 (4.9) |
| Indicated * | 168 (3.7) | 104 (2.3) | 100 (2.2) |
| Spontaneous | 99 (2.2) | 109 (2.4) | 117 (2.6) |
| PTB < 34 weeks * | 53 (1.2) | 33 (0.7) | 36 (0.8) |
| Indicated * | 40 (0.9) | 22 (0.5) | 21 (0.5) |
| Spontaneous | 13 (0.3) | 11 (0.2) | 15 (0.3) |
| Birthweight (g) * | 3147 ± 497 | 3250 ± 463 | 3268 ± 466 |
| Study Subgroups | Risk for PTB (<37 Weeks) in 3rd Delivery | Risk for Spontaneous PTB (<37 Weeks) in 3rd Delivery | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Delivery | 2nd Delivery | N (%) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) |
| Term | Term | 4038 (90.3) | 140 (3.5) | reference | reference | 65 (1.6) | reference | reference |
| Preterm | Term | 221 (4.9) | 24 (10.9) | 3.39 (2.15–5.35) | 3.08 (1.92–4.92) | 11 (5.0) | 3.20 (1.66–6.16) | 3.11 (1.61–6.03) |
| Term | Preterm | 167 (3.7) | 27 (16.2) | 5.37 (3.44–8.38) | 5.64 (3.59–8.84) | 20 (12.0) | 8.32 (4.91–14.09) | 8.58 (5.05–14.59) |
| Preterm | Preterm | 46 (1.0) | 26 (56.5) | 36.19 (19.72–66.40) | 38.24 (20.63–70.85) | 21 (45.7) | 37.88 (20.49–70.04) | 51.90 (27.28–97.71) |
| Study Subgroups | Risk for Spontaneous PTB (<37 Weeks) in 3rd Delivery | Risk for Spontaneous PTB (<34 Weeks) in 3rd Delivery | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Delivery | 2nd Delivery | N (%) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) |
| Term | Term | 4038 (90.3) | 65 (1.6) | reference | reference | 9 (0.2) | reference | reference |
| sPTD | Term | 75 (1.7) | 4 (5.3) | 2.14 (0.76–5.94) | 3.28 (1.15–9.33) | 1 (1.3) | 4.23 (0.55–32.59) | 6.33 (0.79–50.74) |
| Term | sPTD | 87 (1.9) | 16 (18.4) | 9.56 (5.37–17.02) | 14.55 (7.98–26.53) | 1 (1.1) | 3.63 (0.47–27.91) | 5.18 (0.65–41.37) |
| sPTD | sPTD | 15 (0.3) | 7 (46.7) | 34.58 (12.32–97.04) | 57.74 (20.21–164.95) | 1 (6.7) | 22.67 (2.79–184.28) | 31.41 (3.71–265.79) |
| Study Subgroups | Risk for Spontaneous PTB (<37 Weeks) in 3rd Delivery | Risk for Spontaneous PTB (<34 Weeks) in 3rd Delivery | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Delivery | 2nd Delivery | N (%) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) | Rate of PTB n (%) | Crude OR (95% C.I) | Adjusted OR * (95% C.I) |
| Term | Term | 4038 (90.3) | 65 (1.6) | reference | reference | 9 (0.2) | reference | reference |
| iPTB | Term | 146 (3.3) | 7 (4.8) | 1.93 (0.88–4.22) | 3.03 (1.35–6.77) | 1 (0.7) | 2.12 (0.27–16.26) | 3.31 (0.42–26.31) |
| Term | iPTB | 80 (1.8) | 4 (5.0) | 1.99 (0.71–5.54) | 3.26 (1.15–9.21) | 0 (0) | - | - |
| iPTB | iPTB | 15 (0.3) | 5 (33.3) | 19.39 (6.52–57.68) | 32.91 (10.43–103.82) | 1 (6.7) | 22.67 (2.79–184.28) | 43.87 (4.91–391.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiersch, L.; Pasternak, Y.; Melamed, N.; Meshulam, M.; Shashar, R.; Hadar, E.; Aviram, A.; Yogev, Y.; Ashwal, E. The Risk of Preterm Birth in Women with Three Consecutive Deliveries—The Effect of Number and Type of Prior Preterm Births. J. Clin. Med. 2020, 9, 3933. https://doi.org/10.3390/jcm9123933
Hiersch L, Pasternak Y, Melamed N, Meshulam M, Shashar R, Hadar E, Aviram A, Yogev Y, Ashwal E. The Risk of Preterm Birth in Women with Three Consecutive Deliveries—The Effect of Number and Type of Prior Preterm Births. Journal of Clinical Medicine. 2020; 9(12):3933. https://doi.org/10.3390/jcm9123933
Chicago/Turabian StyleHiersch, Liran, Yael Pasternak, Nir Melamed, Moshe Meshulam, Reut Shashar, Eran Hadar, Amir Aviram, Yariv Yogev, and Eran Ashwal. 2020. "The Risk of Preterm Birth in Women with Three Consecutive Deliveries—The Effect of Number and Type of Prior Preterm Births" Journal of Clinical Medicine 9, no. 12: 3933. https://doi.org/10.3390/jcm9123933
APA StyleHiersch, L., Pasternak, Y., Melamed, N., Meshulam, M., Shashar, R., Hadar, E., Aviram, A., Yogev, Y., & Ashwal, E. (2020). The Risk of Preterm Birth in Women with Three Consecutive Deliveries—The Effect of Number and Type of Prior Preterm Births. Journal of Clinical Medicine, 9(12), 3933. https://doi.org/10.3390/jcm9123933






