Endurance Runners with Intramyocellular Lipid Accumulation and High Insulin Sensitivity Have Enhanced Expression of Genes Related to Lipid Metabolism in Muscle
Abstract
:1. Introduction
2. Research Design and Methods
2.1. Subjects
2.2. Study Design and Measurement of Various Parameters
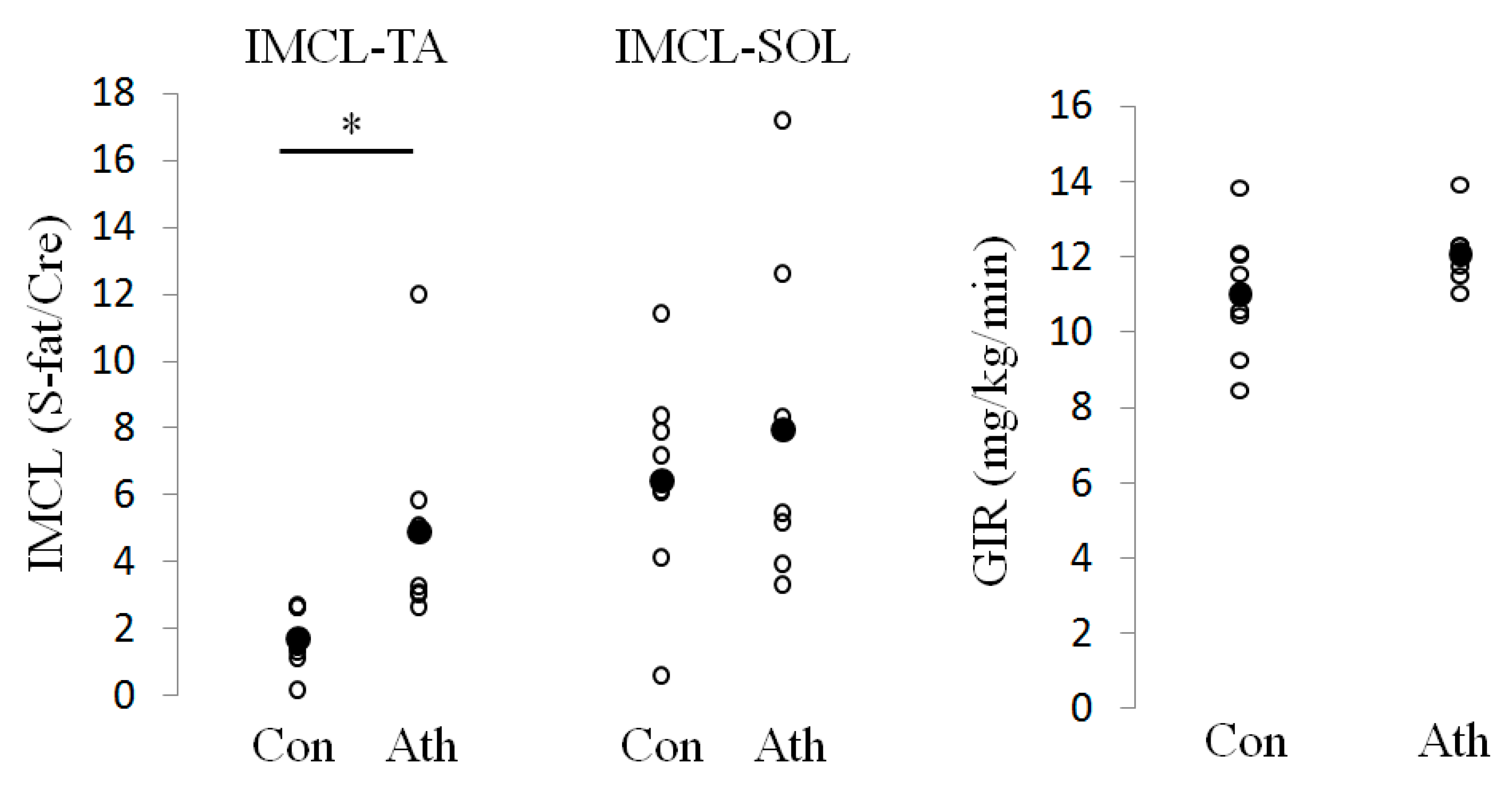
2.3. Proton Magnetic Resonance Spectroscopy
2.4. Hyperinsulinemic Euglycemic Clamp Study
2.5. DNA Microarray Analysis
2.6. DNA Microarray Data Analysis
2.7. qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Subjects
3.2. Hierarchical Clustering Analysis in the Control and Athlete Groups
3.3. Gene Expression Profiles of Muscle in the Control and Athlete Groups
3.4. Gene Set Enrichment Analysis (GSEA) of Skeletal Muscle Tissue in the Control and Athlete Groups
3.5. Gene Expression Analysis Using qRT-PCR in the Control and Athlete Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kakehi, S.; Tamura, Y.; Takeno, K.; Sakurai, Y.; Kawaguchi, M.; Watanabe, T.; Funayama, T.; Sato, F.; Ikeda, S.; Kanazawa, A.; et al. Increased intramyocellular lipid/impaired insulin sensitivity is associated with altered lipid metabolic genes in muscle of high responders to a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 32–40. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, V.B.; Jorett, A.E.; Marchetti, C.M.; Gonzalez, F.; Phillips, S.A.; Ciaraldi, T.P.; Kirwan, J.P. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am. J. Physiol. 2007, 293, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, T.; Paulsen, S.K.; Bruun, J.M.; Ploug, T.; Pedersen, S.B.; Richelsen, B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J. Clin. Endocrinol. Metab. 2010, 95, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Bullen, J.W., Jr.; Lee, J.H.; Kralisch, S.; Fasshauer, M.; Kloting, N.; Niebauer, J.; Schon, M.R.; Williams, C.J.; Mantzoros, C.S. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: Associations with metabolic parameters and insulin resistance and regulation by physical training. J. Clin. Endocrinol. Metab. 2006, 91, 2310–2316. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, D.J.; Parise, G.; Melov, S.; Safdar, A.; Tarnopolsky, M.A. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005, 19, 1498–1500. [Google Scholar] [CrossRef]
- Norrbom, J.; Sundberg, C.J.; Ameln, H.; Kraus, W.E.; Jansson, E.; Gustafsson, T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J. Appl. Physiol. 2004, 96, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003, 546, 851–858. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Van Loon, L.J.; Goodpaster, B.H. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch. 2006, 451, 606–616. [Google Scholar] [CrossRef]
- Teran-Garcia, M.; Rankinen, T.; Koza, R.A.; Rao, D.C.; Bouchard, C. Endurance training-induced changes in insulin sensitivity and gene expression. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Larsson, O.; Jansson, E.; Fischer, H.; Gustafsson, T.; Greenhaff, P.L.; Ridden, J.; Rachman, J.; Peyrard-Janvid, M.; Wahlestedt, C.; et al. Human muscle gene expression responses to endurance training provide a novel perspective on Duchenne muscular dystrophy. FASEB J. 2005, 19, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Knudsen, S.; Rankinen, T.; Koch, L.G.; Sarzynski, M.; Jensen, T.; Keller, P.; Scheele, C.; Vollaard, N.B.; Nielsen, S.; et al. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J. Appl. Physiol. 2010, 108, 1487–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busso, T.; Fluck, M. A mixed-effects model of the dynamic response of muscle gene transcript expression to endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Coffey, V.G.; Carey, A.L.; Ponnampalam, A.P.; Canny, B.J.; Powell, D.; Hawley, J.A. Global gene expression in skeletal muscle from well-trained strength and endurance athletes. Med. Sci. Sports Exerc. 2009, 41, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, M.; Billeter, R.; Hoppeler, H.; Fluck, M. Regulatory gene expression in skeletal muscle of highly endurance-trained humans. Acta Physiol. Scand. 2004, 180, 217–227. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Tamura, Y.; Kakehi, S.; Takeno, K.; Sakurai, Y.; Watanabe, T.; Funayama, T.; Sato, F.; Ikeda, S.; Ogura, Y.; et al. Association between expression of FABPpm in skeletal muscle and insulin sensitivity in intramyocellular lipid-accumulated nonobese men. J. Clin. Endocrinol. Metab. 2014, 99, 3343–3352. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y.; Tamura, Y.; Takeno, K.; Kumashiro, N.; Sato, F.; Kakehi, S.; Ikeda, S.; Ogura, Y.; Saga, N.; Naito, H.; et al. Determinants of intramyocellular lipid accumulation after dietary fat loading in non-obese men. J. Diabetes Investig. 2011, 2, 310–317. [Google Scholar] [CrossRef]
- Sato, F.; Tamura, Y.; Watada, H.; Kumashiro, N.; Igarashi, Y.; Uchino, H.; Maehara, T.; Kyogoku, S.; Sunayama, S.; Sato, H.; et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J. Clin. Endocrinol. Metab. 2007, 92, 3326–3329. [Google Scholar] [CrossRef] [Green Version]
- Takeno, K.; Tamura, Y.; Kawaguchi, M.; Kakehi, S.; Watanabe, T.; Funayama, T.; Furukawa, Y.; Kaga, H.; Yamamoto, R.; Kim, M.; et al. Relation between insulin sensitivity and metabolic abnormalities in japanese men with BMI of 23–25 kg/m2. J. Clin. Endocrinol. Metab. 2016, 101, 3676–3684. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Tanaka, Y.; Sato, F.; Choi, J.B.; Watada, H.; Niwa, M.; Kinoshita, J.; Ooka, A.; Kumashiro, N.; Igarashi, Y.; et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2005, 90, 3191–3196. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Watada, H.; Igarashi, Y.; Nomiyama, T.; Onishi, T.; Takahashi, K.; Doi, S.; Katamoto, S.; Hirose, T.; Tanaka, Y.; et al. Short-term effects of dietary fat on intramyocellular lipid in sprinters and endurance runners. Metabolism 2008, 57, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Strauss, A.; Bacon, S.; Kerege, A.; Harrison, K.; Brozinick, J.T.; Hunerdosse, D.M.; Playdon, M.C.; Holmes, W.; et al. Intramuscular triglyceride synthesis: Importance in muscle lipid partitioning in humans. Am. J. Physiol. Endocrinol. Metab. 2018, 314, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Anderson, M.J.; Carey, A.L.; Newman, D.G.; Bonen, A.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J. Clin. Endocrinol. Metab. 2003, 88, 5444–5451. [Google Scholar] [CrossRef] [Green Version]
- Turban, S.; Hajduch, E. Protein kinase C isoforms: Mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011, 585, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Kano, Y.; Shimegi, S.; Furukawa, H.; Matsudo, H.; Mizuta, T. Effects of aging on capillary number and luminal size in rat soleus and plantaris muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Poole, D.C.; Mathieu-Costello, O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation 1996, 3, 175–186. [Google Scholar] [CrossRef]
- Sullivan, S.M.; Pittman, R.N. Relationship between mitochondrial volume density and capillarity in hamster muscles. Am. J. Physiol. 1987, 252, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.J.; Koopman, R.; Manders, R.; van der Weegen, W.; van Kranenburg, G.P.; Keizer, H.A. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am. J. Physiol. Endocrinol. Metab. 2004, 287, 558–565. [Google Scholar] [CrossRef]
- Polgar, J.; Johnson, M.A.; Weightman, D.; Appleton, D. Data on fibre size in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 19, 307–318. [Google Scholar] [CrossRef]
- Kiens, B.; Roepstorff, C.; Glatz, J.F.; Bonen, A.; Schjerling, P.; Knudsen, J.; Nielsen, J.N. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: Influence of physical activity and gender. J. Appl. Physiol. 2004, 97, 1209–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talanian, J.L.; Holloway, G.P.; Snook, L.A.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am. J. Physiol. 2010, 299, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcotte, L.P.; Richter, E.A.; Kiens, B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am. J. Physiol. 1992, 262, 791–799. [Google Scholar] [CrossRef]
- Nickerson, J.G.; Alkhateeb, H.; Benton, C.R.; Lally, J.; Nickerson, J.; Han, X.X.; Wilson, M.H.; Jain, S.S.; Snook, L.A.; Glatz, J.F.; et al. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J. Biol. Chem. 2009, 284, 16522–16530. [Google Scholar] [CrossRef] [Green Version]
- Holloway, G.P.; Chou, C.J.; Lally, J.; Stellingwerff, T.; Maher, A.C.; Gavrilova, O.; Haluzik, M.; Alkhateeb, H.; Reitman, M.L.; Bonen, A. Increasing skeletal muscle fatty acid transport protein 1 (FATP1) targets fatty acids to oxidation and does not predispose mice to diet-induced insulin resistance. Diabetologia 2011, 54, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Levak-Frank, S.; Radner, H.; Walsh, A.; Stollberger, R.; Knipping, G.; Hoefler, G.; Sattler, W.; Weinstock, P.H.; Breslow, J.L.; Zechner, R. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J. Clin. Invest. 1995, 96, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Vega, R.B.; Huss, J.M.; Kelly, D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000, 20, 1868–1876. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.G. Peroxisome proliferator activated receptor-alpha (PPARalpha) and PPAR gamma coactivator-1alpha (PGC-1alpha) regulation of cardiac metabolism in diabetes. Pediatr. Cardiol. 2011, 32, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Mandard, S.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. Cell. Mol. Life Sci. 2004, 61, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Mandard, S.; Patsouris, D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013, 2013, 613864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supruniuk, E.; Miklosz, A.; Chabowski, A. The implication of PGC-1alpha on fatty acid transport across plasma and mitochondrial membranes in the insulin sensitive tissues. Front. Physiol. 2017, 8, 923. [Google Scholar] [CrossRef] [PubMed]




| Control Group (n = 8) | Athlete Group (n = 7) | |
|---|---|---|
| Age (years) | 21.7 ± 1.4 | 21.6 ± 0.8 |
| Body mass index (kg/m2) | 19.7 ± 1.1 | 20.5 ± 1.1 |
| Body fat (%) | 12 ± 3.5 | 13.3 ± 3.0 |
| Glucose (mg/dL) | 83.4 ± 5.1 | 85.6 ± 7.1 |
| Insulin (μU/mL) | 2.8 ± 1.0 | 4.3 ± 1.2 |
| Free fatty acids (mmol/L) | 0.43 ± 0.16 | 0.49 ± 0.10 |
| Total cholesterol (mg/dL) | 169 ± 17.2 | 208.6 ± 33.3 * |
| High-density lipoprotein cholesterol (mg/dL) | 59.4 ± 11.4 | 59.4 ± 10.9 |
| Triglycerides (mg/dL) | 62.2 ± 31.0 | 65.2 ± 20.3 |
| Hemoglobin A1c (%) | 4.7 ± 0.2 | 4.7 ± 0.2 |
| High molecular weight adiponectin (µg/mL) | 1.81 ± 1.15 | 1.89 ± 1.20 |
| Maximum oxygen uptake (mL/kg/min) | 52.2 ± 6.9 | 58.4 ± 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakehi, S.; Tamura, Y.; Takeno, K.; Ikeda, S.-i.; Ogura, Y.; Saga, N.; Miyatsuka, T.; Naito, H.; Kawamori, R.; Watada, H. Endurance Runners with Intramyocellular Lipid Accumulation and High Insulin Sensitivity Have Enhanced Expression of Genes Related to Lipid Metabolism in Muscle. J. Clin. Med. 2020, 9, 3951. https://doi.org/10.3390/jcm9123951
Kakehi S, Tamura Y, Takeno K, Ikeda S-i, Ogura Y, Saga N, Miyatsuka T, Naito H, Kawamori R, Watada H. Endurance Runners with Intramyocellular Lipid Accumulation and High Insulin Sensitivity Have Enhanced Expression of Genes Related to Lipid Metabolism in Muscle. Journal of Clinical Medicine. 2020; 9(12):3951. https://doi.org/10.3390/jcm9123951
Chicago/Turabian StyleKakehi, Saori, Yoshifumi Tamura, Kageumi Takeno, Shin-ichi Ikeda, Yuji Ogura, Norio Saga, Takeshi Miyatsuka, Hisashi Naito, Ryuzo Kawamori, and Hirotaka Watada. 2020. "Endurance Runners with Intramyocellular Lipid Accumulation and High Insulin Sensitivity Have Enhanced Expression of Genes Related to Lipid Metabolism in Muscle" Journal of Clinical Medicine 9, no. 12: 3951. https://doi.org/10.3390/jcm9123951






