Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Population
2.2. Sampling
2.3. Microbiological Diagnostic Procedures
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
3. Results
3.1. Microbiological Diagnosis
3.2. Electron Microscopy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann. Otol. Rhinol. Laryngol. 2011, 120, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Palade, O.D.; Severin, F.; Toader, M.; Cobzeanu, M.D.; Toader, C. Combined approach for large tumors of the nose and paranasal sinuses—Case report. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2016, 120, 380–383. [Google Scholar] [PubMed]
- Shashy, R.G.; Moore, E.J.; Weaver, A. Prevalence of the chronic sinusitis diagnosis in Olmsted County, Minnesota. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 320–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.C.; Yeh, Y.T.; Lin, C.C.; Huang, C.H.; Liu, H.C.; Chiang, C.P. Clinical Detection of Chronic Rhinosinusitis through Next-Generation Sequencing of the Oral Microbiota. Microorganisms 2020, 6, 959. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, M.; Wagner Mackenzie, B.; Jain, R.; Taylor, M.W.; Biswas, K.; Douglas, R.G. Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease. Clin. Microbiol. Rev. 2017, 30, 321–348. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.; Stokken, J.; Sanford, T.; Aurora, R.; Sindwani, R. A systematic review of the sinonasal microbiome in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 161–166. [Google Scholar] [CrossRef]
- Schubert, S.; Kostrzewa, M. MALDI-TOF MS in the Microbiology Laboratory: Current Trends. Curr. Issues Mol. Biol. 2017, 23, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Dingle, T.C.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Hauser, L.J.; Frank, D.N. The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boeck, I.; Wittouck, S.; Martens, K.; Claes, J.; Jorissen, M.; Steelant, B.; van den Broek, M.F.L.; Seys, S.F.; Hellings, P.W.; Vanderveken, O.M.; et al. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere 2019, 4, e00532-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeller, K.; Herlemann, D.; Schuldt, T.; Ovari, A.; Guder, E.; Podbielski, A.; Kreikemeyer, B.; Olzowy, B. Microbiome and culture based analysis of chronic rhinosinusitis compared to healthy sinus mucosa. Front. Microbiol. 2018, 9, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boase, S.; Foreman, A.; Cleland, E.; Tan, L.; Melton-Kreft, R.; Pant, H.; Hu, F.Z.; Ehrlich, G.D.; Wormald, P.J. The microbiome of chronic rhinosinusitis: Culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 2013, 13, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Bleier, B.S.; Wei, Y. Current understanding of the acute exacerbation of chronic rhinosinusitis. Front. Cell Infect. Microbiol. 2019, 9, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, I. Bacteriology of chronic sinusitis and acute exacerbation of chronic sinusitis. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1099–1101. [Google Scholar] [CrossRef] [Green Version]
- Wolcott, R.; Costerton, J.W.; Raoult, D.; Cutler, S.J. The polymicrobial nature of biofilm infection. Clin. Microbiol. Infect. 2013, 19, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, K.A.; Eskeland, O.; Fridrich-Aas, K.; Orszagh, V.C.; Bachmann-Harildstad, G.; Burum-Auensen, E. Bacterial biofilms in patients with chronic rhinosinusitis: A confocal scanning laser microscopy study. Rhinology 2014, 52, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.J.; Jung, S.M.; Lee, H.N.; Kim, H.G.; Chung, J.H.; Jeong, J.H. Treatment strategy for odontogenicsinusitis. Am. J. Rhinol. Allergy 2020, 1945892420946969. [Google Scholar] [CrossRef]
- Yassin-Kassab, A.; Bhargava, P.; Tibbetts, R.J.; Griggs, Z.H.; Peterson, E.I.; Craig, J.R. Comparison of bacterial maxillary sinus cultures between odontogenic sinusitis and chronicrhinosinusitis. Int. Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zirk, M.; Dreiseidler, T.; Pohl, M.; Rothamel, D.; Buller, J.; Peters, F.; Zöller, J.E.; Kreppel, M. Odontogenic sinusitis maxillaris: A retrospective study of 121 cases with surgical intervention. J. Craniomaxillofac. Surg. 2017, 45, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Saibene, A.M.; Vassena, C.; Pipolo, C.; Trimboli, M.; De Vecchi, E.; Felisati, G.; Drago, L. Odontogenic and rhinogenic chronic sinusitis: A modern microbiological comparison. Int. Forum Allergy Rhinol. 2016, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Whiley, R.A.; Hall, L.M.; Hardie, J.M.; Beighton, D. A study of small-colony, beta-haemolytic, Lancefield group C streptococci within the anginosus group: Description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int. J. Syst. Bacteriol. 1999, 49, 1443–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.K.; Kerschner, J.E. Streptococcus milleri: An organism for head and neck infections and abscess. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankhethoa, N.M.; Prescott, C.A. Significance of Streptococcus milleri in acute rhinosinusitis with complications. J. Laryngol. Otol. 2008, 122, 810–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, S.; Yazar, U.; Cansu, A.; Kul, S.; Kaya, S.; Özdoğan, E.B. Is sinusitis innocent?--Unilateral subdural empyema in an immunocompetent child. Indian J. Pediatr. 2015, 82, 1061–1064. [Google Scholar] [CrossRef]
- Mantovani, K.; Bisanha, A.A.; Demarco, R.C.; Tamashiro, E.; Martinez, R.; Anselmo-Lima, W.T. Maxillary sinuses microbiology from patients with chronic rhinosinusitis. Braz. J. Otorhinolaryngol. 2010, 76, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Danielides, V.; Nousia, C.S.; Gesouli, E.; Papakostas, V.; Milionis, H.J.; Skevas, A. Recurrent facial pain due to Pseudo monas aeruginosa sinusitis. Rhinology 2002, 40, 226–228. [Google Scholar]
- Drago, L.; Vassena, C.; Saibene, A.M.; Del Fabbro, M.; Felisati, G. A case of coinfection in a chronic maxillary sinusitis of odontogenic origin: Identification of Dialister pneumosintes. J. Endod. 2013, 39, 1084–1087. [Google Scholar] [CrossRef]
- Kogure, M.; Suzuki, H.; Ishiguro, S.; Ueda, A.; Nakahara, T.; Tamai, K.; Notake, S.; Shiotani, S.; Umemoto, T.; Morishima, I.; et al. Dialister pneumo sintes bacteremia caused by dental caries and sinusitis. Intern. Med. 2015, 54, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davin-Regli, A.; Pagès, J.M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmousa, R.; Dadashzadeh, R.; Ahangarkani, F.; Rezai, M.S. Frequency of bacterial agents isolated from patients with chronic sinusitis in Northern Iran. Glob. J. Health Sci. 2015, 8, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombaux, P.; Gigi, J.; Hamoir, M.; Eloy, P.; Bertrand, B. Bacteriology of chronic sinusitis: The bulla ethmoidalis content. Rhinology 2002, 40, 18–23. [Google Scholar] [PubMed]
- Brook, I. The role of anaerobic bacteria in sinusitis. Anaerobe 2006, 12, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.A. Gram-positive anaerobic cocci. Clin. Microbiol. Rev. 1998, 11, 81–120. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Kalcioglu, M.T.; Durmaz, B.; Aktas, E.; Ozturan, O.; Durmaz, R. Bacteriology of chronic maxillary sinusitis and normal maxillary sinuses: Using culture and multiplex polymerase chain reaction. Am. J. Rhinol. 2003, 17, 143–147. [Google Scholar] [CrossRef]
- Yassin, N.A.; Ahmad, A.M. Incidence and resistotyping profiles of Bacillus subtilis isolated from Azadi Teaching Hospital in Duhok City, Iraq. Mater. Sociomed. 2012, 24, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Argemi, X.; Hansmann, Y.; Riegel, P.; Prévost, G. Is Staphylococcus lugdunensis significant in clinicals amples? J. Clin. Microbiol. 2017, 55, 3167–3174. [Google Scholar] [CrossRef] [Green Version]
- Bieber, L.; Kahlmeter, G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin. Microbiol. Infect. 2010, 16, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R. Matrix-assisted laser desorption ionization-time of flight mass spectrometry in clinical microbiology. Clin. Infect. Dis. 2013, 57, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, P.C.; Lazarus, R.; Protheroe, A.; Milford, C.; Bowler, I.C. Acute necrotizing sinusitis caused by Staphylococcus lugdunensis. J. Clin. Microbiol. 2011, 49, 2740–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskandar, S.; Miller-Ensminger, T.; Voukadinova, A.; Wolfe, A.J.; Putonti, C. Draft genome sequence of Corynebacterium aurimucosum UMB7769, isolated from the female urinary tract. Microbiol. Resour. Announc. 2020, 9, e00391-20. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Mullany, P.; Roberts, A.P. Draft genome sequence of Eggerthia catenaformis strain MAR1 isolated from saliva of healthy humans. Genome Announc. 2017, 5, e00638-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foronda, C.; Calatrava, E.; Casanovas, I.; Martín-Hita, L.; Navarro-Marí, J.M.; Cobo, F. Eggerthia catenaformis bacteremia in a patient with an odontogenic abscess. Anaerobe 2019, 57, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Kordjian, H.H.; Schultz, J.D.; Rosenvinge, F.S.; Møller, J.; Pedersen, R.M. First clinical description of Eggerthia catenaformis bacteremia in a patient with dental abscess. Anaerobe 2015, 35, 38–40. [Google Scholar] [CrossRef]
- Horiuchi, A.; Kokubu, E.; Warita, T.; Ishihara, K. Synergistic biofilm formation by Parvimonas micra and Fusobacterium nucleatum. Anaerobe 2020, 62, 102100. [Google Scholar] [CrossRef]
- Khan, M.S.; Ishaq, M.; Hinson, M.; Potugari, B.; Rehman, A.U. Parvimonas micra bacteremia in a patient with colonic carcinoma. Caspian J. Intern. Med. 2019, 10, 472–475. [Google Scholar] [CrossRef]
- Toskala, E.; Rautiainen, M. Electron microscopy assessment of the recovery of sinus mucosa after sinus surgery. Acta Otolaryngol. 2003, 123, 954–959. [Google Scholar] [CrossRef]
- Galli, J.; Calo, L.; Ardito, F.; Imperiali, M.; Bassotti, E.; Passali, G.C.; La Torre, G.; Paludetti, G.; Fadda, G. Damage to ciliated epithelium in chronic rhinosinusitis: What is the role of bacterial biofilms? Ann. Otol. Rhinol. Laryngol. 2008, 117, 902–909. [Google Scholar] [CrossRef] [PubMed]



| Gender Distribution | ||||
|---|---|---|---|---|
| Gender | Male | Female | ||
| No. of Patients | 11 | 21 | ||
| Age Distribution | ||||
| No. of Patients | CRSsNP (Primary CRS Diffuse Non-Type 2) | CRSwNP (Primary CRS Diffuse Type 2) | Odontogenic CRS (Secondary CRS Unilateral) | Sphenoid CRS |
| 32 | 5 | 11 | 13 | 3 |
| Bacterial Species Isolated | ||||
| Species identified | Gram Staining Tinctorial Affinity | Metabolism | Biofilm | |
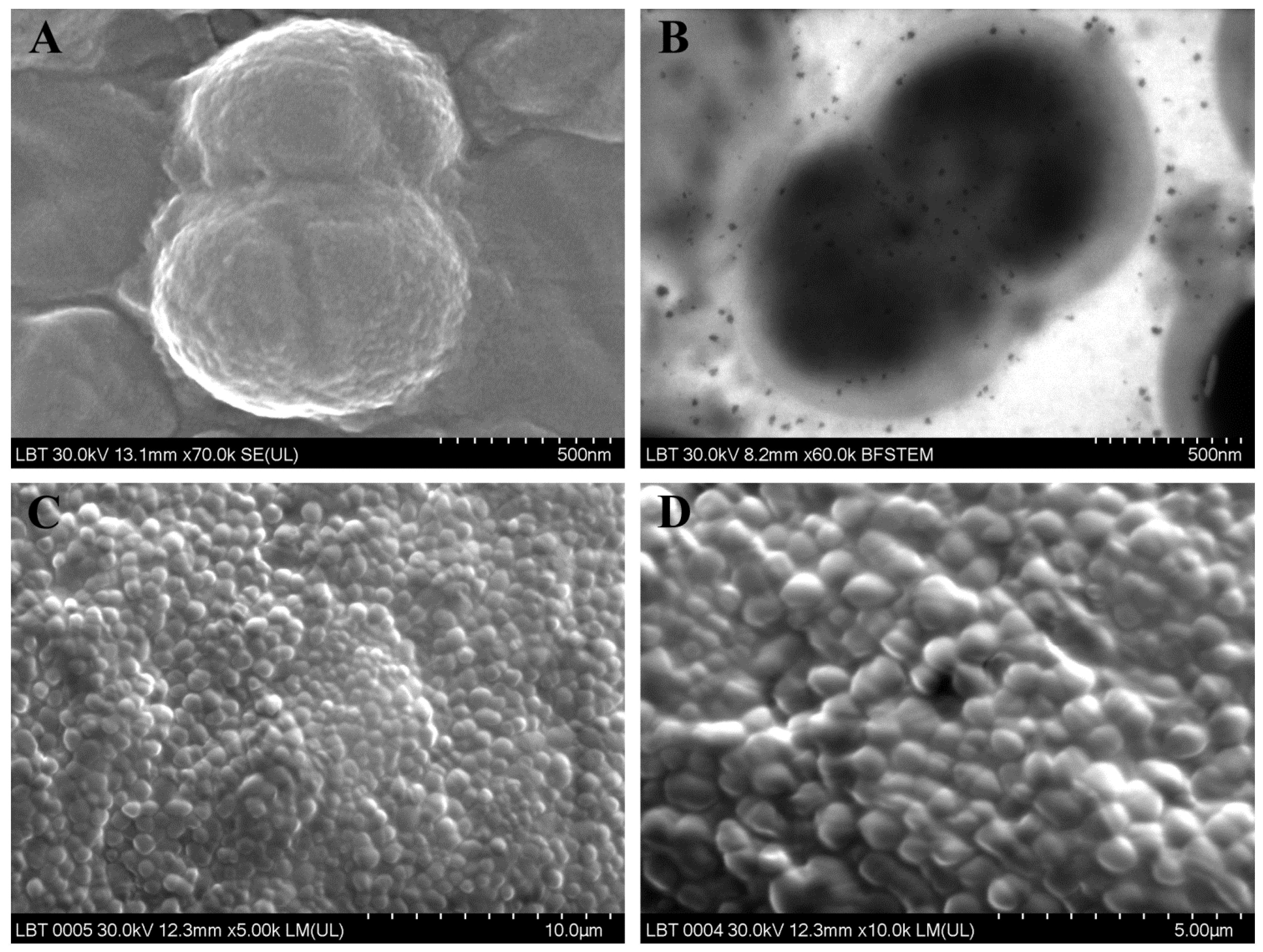
| CRSsNP (Primary CRS diffuses non-type 2) | Enterococcus faecalis (Figure 1A,B) | G+ | Facultatively anaerobic | Absent |
| sensitive to ampicillin, ciprofloxacin, vancomycin | ||||
| Pseudomonas aeruginosa | G− | Aerobic | Present | |
| Staphylococcus aureus (Figure 2A) | G+ | Aerobic | ||
| Pseudomonas: resistant to sulfamethoxazole-trimethoprim, amoxicillin-clavulanate, cefuroxime; sensitive to colistin, ciprofloxacin, ceftazidime. Staphylococcus: methicillin-sensitive. | ||||
| CRSwNP (Primary CRS diffuse type 2) | Staphylococcus aureus | G+ | Aerobic | Present |
| Bacillus subtilis | G+ | Aerobic | ||
| Staphylococcus: resistant to oxacilin, clindamycin; sensitive to: ciprofloxacin, gentamicin, sulfamethoxazole-trimethoprim | ||||
| Staphylococcus lugdunensis | G+ | Aerobic | Absent | |
| sensitive to: oxacillin, ciprofloxacin, gentamicin, sulfamethoxazole-trimethoprim | ||||
| Klebsiella pneumoniae (Figure 2C) | G− | Facultatively anaerobic | Present | |
| resistant to ampicillin; sensitive to: amoxicillin-clavulanate, ciprofloxacin, gentamicin, sulfamethoxazole-trimethoprim | ||||
| Staphylococcus aureus (2 samples) * | G+ | Aerobic | Present in 1 of 2 samples | |
| methicillin-sensitive | ||||
| Finegoldia magna (Figure 2D) | G+ | Anaerobic | Absent | |
| Prevotella oris | G− | Anaerobic | Present | |
| Prevotella buccae | G− | Anaerobic | ||
| Staphylococcus lugdunensis | G+ | Anaerobic | ||
| Staphylococcus: methicillin-sensitive | ||||
| Haemophilus influenzae | G− | Facultatively anaerobic | Absent | |
| sensitive to: ampicillin, amoxicillin-clavulanate, sulfamethoxazole-trimethoprim | ||||
| Streptococcus constellatus subsp. pharyngis | G+ | Anaerobic | Absent | |
| Bacteroides pyogenes | G− | Anaerobic | ||
| Streptococcus: sensitive to clindamycin, erythromycin | ||||
| Odontogenic CRS (Secondary CRS unilateral) | Enterobacter aerogenes | G− | Facultatively anaerobic | Present |
| Finegoldia magna | G+ | Anaerobic | ||
| Enterobacter: resistant to ampicillin, amoxicillin-clavulanate; sensitive to: cephalosporins, gentamicin, sulfamethoxazole-trimethoprim | ||||
| Dialister pneumosintes | G− | Anaerobic | Absent | |
| Streptococcus constellatus subsp. pharyngis (2 samples) (Figure 2B) | G+ | Anaerobic | Absent | |
| sensitive to clindamycin, erythromycin | ||||
| Staphylococcus lugdunensis | G+ | Aerobic | Absent | |
| Prevotella buccae | G− | Anaerobic | ||
| Staphylococcus: sensitive to: ciprofloxacin, gentamicin, sulfamethoxazole-trimethoprim | ||||
| Staphylococcus aureus | G+ | Aerobic | Present | |
| methicillin-sensitive | ||||
| Klebsiella pneumoniae | G− | Facultatively anaerobic | Present | |
| Streptococcus constellatus subsp. pharyngis | G+ | Facultatively anaerobic | ||
| Parvimonas micra (Figure 1C,D) | G+ | Anaerobic | ||
| Streptococcus: sensitive to clindamycin, erythromycin | ||||
| Staphylococcus lugdunensis | G+ | Aerobic | Present | |
| Streptococcus constellatus subsp. pharyngis * | G+ | Anaerobic | ||
| Staphylococcus: resistant to oxacillin, ciprofloxacin, gentamicin; sensitive to: sulfamethoxazole-trimethoprim, clindamycin | ||||
| Corynebacterium aurimucosum (Figure 2E) | G+ | Aerobic | Absent | |
| Eggerthia catenaformis (Figure 2F) | G+ | Anaerobic | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeican, I.I.; Barbu Tudoran, L.; Florea, A.; Flonta, M.; Trombitas, V.; Apostol, A.; Dumitru, M.; Aluaș, M.; Junie, L.M.; Albu, S. Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania. J. Clin. Med. 2020, 9, 3973. https://doi.org/10.3390/jcm9123973
Jeican II, Barbu Tudoran L, Florea A, Flonta M, Trombitas V, Apostol A, Dumitru M, Aluaș M, Junie LM, Albu S. Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania. Journal of Clinical Medicine. 2020; 9(12):3973. https://doi.org/10.3390/jcm9123973
Chicago/Turabian StyleJeican, Ionuț Isaia, Lucian Barbu Tudoran, Adrian Florea, Mirela Flonta, Veronica Trombitas, Anda Apostol, Mihai Dumitru, Maria Aluaș, Lia Monica Junie, and Silviu Albu. 2020. "Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania" Journal of Clinical Medicine 9, no. 12: 3973. https://doi.org/10.3390/jcm9123973







