Abstract
L-arginine and its derivatives, asymmetric and symmetric dimethylarginine (ADMA and SDMA) and L-homoarginine, have emerged as cardiovascular biomarkers linked to cardiovascular outcomes and various metabolic and functional pathways such as NO-mediated endothelial function. Cellular uptake and efflux of L-arginine and its derivatives are facilitated by transport proteins. In this respect the cationic amino acid transporters CAT1 and CAT2 (SLC7A1 and SLC7A2) and the system y+L amino acid transporters (SLC7A6 and SLC7A7) have been most extensively investigated, so far, but the number of transporters shown to mediate the transport of L-arginine and its derivatives is constantly increasing. In the present review we assess the growing body of evidence regarding the function, expression, and clinical relevance of these transporters and their possible relation to cardiovascular diseases.
1. Introduction
Cardiovascular diseases (CVD) remain the primary cause of mortality worldwide [1]. According to the World Health Organization (WHO), cardiovascular diseases are estimated to cause 17.9 million premature deaths worldwide annually. Inflammation, oxidative stress, and endothelial dysfunction which are strongly associated with various classical and non-classical CVD risk factors, are on the front line in the onset and progression of atherosclerosis and CVD in general.
Numerous independent risk markers for adverse cardiovascular outcomes have been identified so far. Nonetheless, only a few of them are considered established risk factors possibly suitable for therapeutic interventions. L-arginine-nitric oxide (NO) signaling plays a critical role in vascular function [2], a low plasma concentration of L-arginine results in impaired L-arginine-NO signaling and may promote endothelial dysfunction [3,4]. Therefore, L-arginine supplementation has been investigated as a possible therapeutic approach [5,6]. However, while preclinical and short-term interventions have shown promising results [7,8,9], the success remains somewhat limited in terms of long-term outcome and survival benefit so far [10]. Changes in the L-arginine plasma concentration have been associated with effects on nitric oxide synthase (NOS) activity that would not be expected based on the known affinity of endothelial NOS (eNOS/NOS3) for L-arginine. This has also been referred to as an “L-arginine paradox” [11], hinting at possible effects of inhibitors, such as asymmetric dimethylarginine (ADMA), or some form of compartmentalization [12,13,14].
Moreover, while L-arginine and its endogenous homologue L-homoarginine, which is also a substrate of nitric oxide synthase (NOS), have been investigated as a protective risk marker, the methylated L-arginine derivatives asymmetric and symmetric dimethylarginine (ADMA and symmetric dimethylarginine (SDMA)) have been studied as risk markers, which may impair L-arginine dependent pathways (for reviews see [15,16]). These markers show several interrelations of their biochemical pathways, but partly rather divergent (patho-)physiological associations with clinical outcomes.
In addition to the well-established role of L-arginine as a substrate for NO-mediated signaling [2], experimental and epidemiological data summarized in the following section indicate strong associations of its derivatives ADMA, SDMA, and L-homoarginine with cardiovascular function and cardiovascular outcomes. An understanding of the regulation of their plasma concentrations and homeostasis is essential to understand the interrelation and pathophysiological properties of these markers.
Vallance et al. demonstrated that accumulation of ADMA in renal failure or infusion of ADMA in healthy volunteers may impair NO-synthesis and endothelial function in renal failure [17]. Subsequently, ADMA was also found to be associated with hypertension [18,19]. Even though elevated plasma ADMA levels were reported in patients with chronic kidney diseases (CKD) [17], the role of impaired renal function in ADMA metabolism remains partly elusive [20,21]. Given the possible adverse effects of ADMA on endothelial (i.e., vascular) function and survival [22,23,24], an efficient ADMA clearance may represent a relevant mechanism to counteract its harmful effects.
ADMA is eliminated through intracellular enzymatic degradation mainly by dimethylarginine dimethylaminohydrolase 1 (DDAH1) [23,25], alanine-glyoxylate aminotransferase 2 (AGXT2) [26], and possibly some other mechanisms such as acetylation [27] and by renal excretion [28]; hence cellular uptake is essential for its degradation. Moreover, the important role of the liver in the elimination of ADMA may not only be attributable to the metabolism of ADMA, but also to transport protein-mediated excretion into the bile [29]. Despite its structural similarity to ADMA, SDMA appears not to directly interfere with NO-synthesis and endothelial function at physiological concentrations. Still, SDMA is independently associated with total mortality and adverse cardiovascular outcomes (discussed elsewhere in more detail [30]), leaving possible causal mechanisms to be elucidated. The independent association of L-homoarginine and renal function was found to be inverse to that of ADMA and SDMA [31,32,33,34,35]. L-homoarginine was also described to be a weak substrate of NOS and inhibitor of arginases. However, while biologically possible, these putative pathomechanisms may not completely explain the observations.
Moreover, as also detailed further below and evident from the data presented in Table 1, L-arginine and its derivatives ADMA, SDMA and L-homoarginine appear to be differentially handled in renal tubular cells, despite having relatively similar chemical properties, leading to marked increases and decreases, respectively, in their urinary concentration. Taken together, these findings suggest that distinct transport mechanisms for L-arginine and its related metabolites constitute a necessary prerequisite for compartmentalization and the differential handling and “uses” of these compounds by organs and tissues.

Table 1.
Physiological characteristics of L-arginine and its derivatives.
It is the focus of this review to provide insights into the transport of L-arginine and its derivatives. The following sections aim to provide an update on the transport protein-mediated cellular uptake and release of L-arginine and its derivatives, providing key characteristics of the major transport proteins involved, and, where available evidence linking these transport proteins to human disease with a special focus on CVD.
2. Transporter Families Shown to Transport L-Arginine and/or Its Derivatives
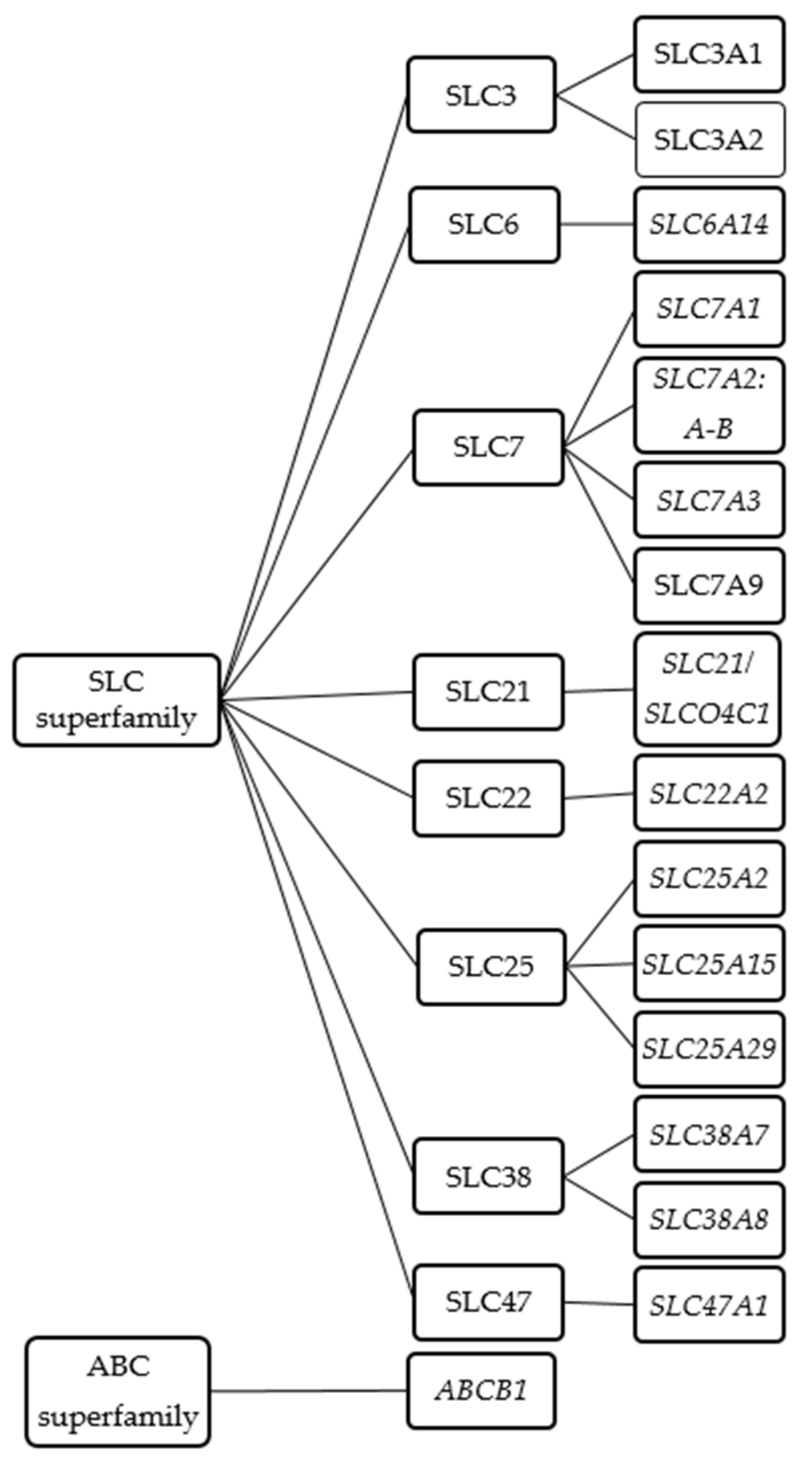
As cationic amino acids, L-arginine and its derivatives do not readily diffuse across cell membranes but rather require some protein-mediated transport mechanism. In humans, at least 18 transporters are considered to mediate the exchange of L-arginine and its derivatives across the cell membrane or between the cytoplasm and cellular organelles such as the mitochondria or lysosomes. These transporters mostly belong to the solute carrier (SLC) superfamily. These include SLC3, SLC6, SLC7, SLCO/SLC21, SLC22, SLC25, SLC38, SLC47 (Figure 1 and Table 2). In addition, members of the adenosine triphosphate (ATP)-binding cassette (ABC) superfamily may also contribute to the cellular exchange of L-arginine and its derivatives (Figure 1 and Table 2). For practical reasons the order of presentation of the respective transporters will follow the transporter nomenclature rather than their biological function.
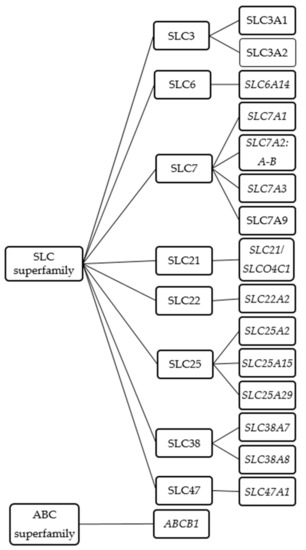
Figure 1.
Schematic diagram of known L-arginine and its derivatives transporters.

Table 2.
Nomenclature of transporters of L-arginine and its derivatives.
2.1. The Solute Carrier (SLC) Superfamily
The SLC transporters (~400 genes) in mammalian cells consist of 65 distinct families, grouped based on their amino acid sequence (primary structure) and their transport function (a systematic record is provided by http://slc.bioparadigms.org/).
2.1.1. SLC6 Family
The SLC6 family is the family of the sodium- and chloride-dependent neurotransmitter transporters. To date about 20 different SLC6 genes have been identified in the human genome [36] that can be further classified into several branches (gamma amino butyric acid transporter branch, the monoamine transporter branch and the amino acid transporter branches 1 and 2) based upon their substrate specificity and sequence similarity [36]. The SLC6 family transport proteins accept a wide range of substrates including neurotransmitters, proteinogenic amino acids, betaine, taurine and creatine. Within this family, the neurotransmitter transporters were initially identified, and thus, it is recognized as the family of neurotransmitter sodium symporters (NSS) or the Na+/Cl−-dependent transporter family [37].
SLC6A14 (ATB0,+)
ATB0,+ (Amino acid Transporter responsible for the activity of system B0,+), encoded by the gene SLC6A14 (Table 2), was functionally identified by Kekuda et al., and cloned from human choriocarcinoma and colon carcinoma [38]. Sloan et al. showed that the expression of SLC6A14 mRNA was significantly higher in human lung and trachea than in other organs [39]. In addition, this sodium- and chloride-dependent neutral and basic amino acid transporter is expressed in pancreas, pituitary, skeletal muscle, placenta, human colon carcinoma cell lines [40,41], and intestine, colon, and kidney proximal tubules [39], where it is located in the luminal membrane [42].
This transporter recognizes neutral and cationic amino acids, excluding aspartate and glutamate [43]. It has some unique characteristics, which differ from other amino acid transporters. The transport activity is dependent on Na+/Cl−, highly concentrative, electrogenic [39], and influenced by the membrane potential [44,45]. So far, ATB0,+ is the only member of this family that has been identified to transport L-arginine. Studies in human bronchial epithelial cells reported KM values of 80 μM for ATB0,+-mediated L-arginine uptake, which most likely is in the range of physiological relevance. The KM value for L-arginine uptake by human ATB0,+ when expressed in Xenopus oocytes was reported to be 104 μM [39]. Issues with regard to the interpretation of KM values obtained in different experimental settings will be addressed in the conclusion.
The SLC6A14 gene has been linked to several diseases (e.g., obesity, ulcerative colitis, colon cancer, breast cancer, and cervical cancer) [46,47,48,49,50]. A meta-analysis of large genome-wide association studies (GWAS) identified the SLC6A14 gene as a potential secondary modifier of cystic fibrosis (CF) comorbidities [51]. A recent study demonstrated that ATB0,+ plays a crucial role in L-arginine transport across the airway epithelial membrane [52]. In this ex vivo study, it was shown that inhibition of the ATB0,+ function in primary human respiratory cell cultures together with the SLc6a14 gene knockout in mice resulted in increased L-arginine concentration in the airway surface liquid.
Interestingly, ATB0,+ expresses functional characteristics that may promote tumor growth: (1) Its ability to transport a large array of amino acids involved in various metabolic pathways vital for tumor growth. (2) Its capacity to facilitate amino acid uptake in a highly concentrative manner. Indeed, overexpression of SLC6A14 has been reported in various cancers (e.g., colon cancer, breast cancer, pancreatic cancer, and cervical cancer) [47,48,50,53]. SLc6a14-knockout mice have been generated which do not express any visible physical or metabolic phenotype. However, development and growth of breast cancer were notably suppressed in a murine mammary tumor virus-polyoma middle tumor-antigen (MTV-PyMT) model deficient in SLc6a14 as compared to MTV-PyMT mice expressing SLc6a14 [54], indicating the potential tumor-promoting activity of ATB0,+ in the mammary gland. However, until now, no studies have been undertaken to investigate whether L-arginine derivatives (e.g., ADMA, SDMA, and L-homoarginine) are also recognized by this transporter. So far, there is no evidence for its involvement in NO-signaling or CVD.
2.1.2. SLC7 Family
The SLC7 family (cationic amino acid transporter/glycoprotein-associated family) consists of two subgroups of transport proteins, cationic amino acid transporters (CATs) and heterodimeric (glycoprotein-associated) amino acid transporters (HATs) [55]. CATs are relatively selective for L-arginine and related compounds, including L-lysine and L-ornithine, whereas HATs have a broad(er) spectrum of substrates also including leucine, glutamine, tryptophan [56]. From the SLC7 family, CATs have been most extensively studied as a transport system for L-arginine and its derivatives. Five members of the CAT family have been cloned, so far. The transport is sodium- and pH-independent [57]. Several CATs have been shown to be sensitive to trans-stimulation [58]. CATs facilitate substrate accumulation against a concentration gradient because of a sensitivity to the membrane potential [59].
SLC7A1 (CAT1)
Of the five CATs proteins, CAT1, a high-affinity electrogenic transporter, was the first to be described at a molecular level. It was initially cloned by Albritton et al. in search of the host cell protein accountable for infection by the murine ecotropic leukaemia virus (MuLV [60]). The SLC7A1 gene encoding human CAT1, was mapped to chromosome 13q12-q14. Excluding the adult’s liver and lacrimal gland [61,62], relevant CAT1 expression can be observed in almost every cell or tissue, albeit at different expression levels (Table 3). CAT1 expression was shown to be modulated by several factors which include insulin, angiotensin II, hormones, nutrients, inflammatory cytokines, and amino acid starvation [63].

Table 3.
An overview of tissue distribution of L-arginine and its derivatives transporters on selected organs and tissues (based on mRNA and protein expression).
By expression in Xenopus oocytes, it was shown that CAT1 facilitates the transport of cationic amino acids (CAAs) [64]. CAT1 transport activities depend on three different factors: concentration gradient, chemical gradient, and membrane potential [65]. CATs are sensitive to trans-stimulation, with CAT1 being the most pronounced [55,66,67]. This feature enables CATs to transport L-arginine and its derivatives both into and out of cells (Figure 2), leading to an exchange of cationic amino acids across the two sides of the membrane which depends on the presence of substrate on either side of the membrane (for review see [66]). Trans-stimulation increases the complexity of in vivo transport kinetics as the intra- and extracellular concentrations of the exchange partners and their respective transport mechanisms have to be considered as well as factors affecting the dynamic equilibrium of L-arginine and its derivatives across cellular membranes. The transport process is also electrogenic, involving the net movement of a positive charge. Based on data from in vitro studies it has been suggested that CAT1 is one major determinant of the plasma levels of L-arginine and its derivatives. CAT1 has been reported to be predominantly expressed in endothelial cells and is assumed to be responsible for 70–95% of L-arginine uptake [68].
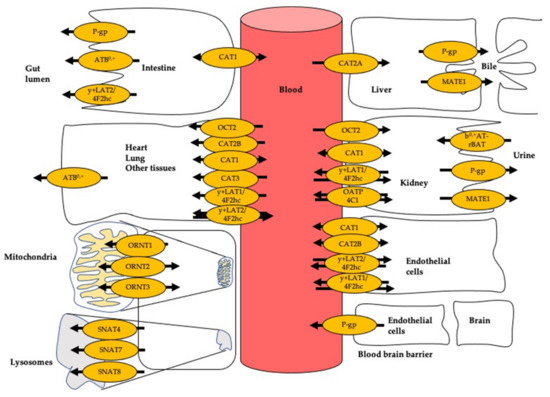
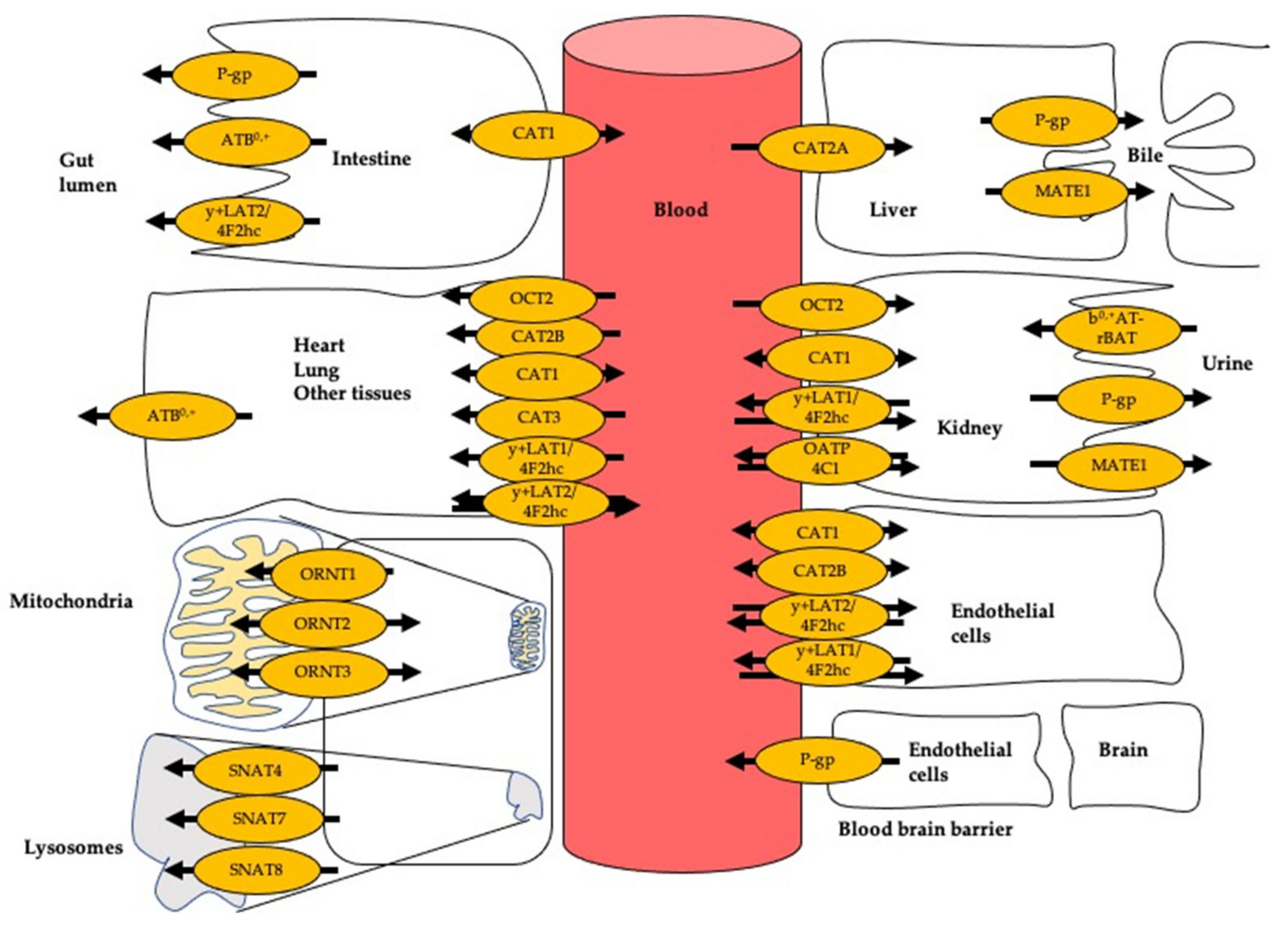
Figure 2.
Transport of L-arginine and its derivatives in different cell types. Arrow indicates the direction of transport. ATB0,+: amino acid transporter responsible for the activity of system B0,+; CAT1: cationic amino acid transporter 1; CAT2A and CAT2B: cationic amino acid transporters 2A and 2B; MATE1: multidrug and toxin extrusion protein 1; OATP4C1: organic anion-transporting polypeptide 4C1; OCT2: organic cation transporter 2; ORNT1: ornithine transporter 1; ORNT2: ornithine transporter 2; ORNT3: ornithine transporter 3; P-gp: P-glycoprotein; SNAT7: sodium-coupled neutral amino acid transporter 4; SNAT7: sodium-coupled neutral amino acid transporter 7; SNAT8: sodium-coupled neutral amino acid transporter 8; y+LAT1: system y+L amino acid transporter 1; 4F2hc: 4F2 cell surface antigen heavy chain; y+LAT2: system y+L amino acid transporter 2.
CAT1 has a distinct feature that may enable this protein to play a significant role in endothelial function: it colocalizes with eNOS in endothelial cell caveolae. This colocalization may enable a direct local transfer of extracellular L-arginine by CAT1 to membrane-bound eNOS, thus making CAT1 a potentially selective supplier of L-arginine for eNOS [69]. CAT1 has mainly been studied as a L-arginine transporter, with early inhibition data indicating a likely role in the transport of ADMA and SDMA as well [70]. As a high-affinity transporter, CAT1 transports L-arginine with apparent KM values in the range of 100 to 519 μM, depending on the model used [71,72]. In a human embryonic kidney (HEK) cell model the apparent KM values for CAT1-mediated L-arginine influx was reported to be 519 μM and 183 and 175 μM for ADMA and L-homoarginine, respectively [71,73]. When interpreting the KM values for the different substrates, their plasma or tissue concentrations have to be considered as well. Because in relation to typical plasma concentrations of 100 µM and 0.5 µM for L-arginine and ADMA, respectively, more L-arginine than ADMA may be transported by this transporter, in absolute as well as in relative terms, despite the transporter’s lower KM for ADMA as compared to L-arginine. No direct studies have been reported for SDMA, so far, but inhibition of L-arginine transport by SDMA has competitive characteristics, indicating SDMA is a substrate as well [70,71]. The uptake of L-arginine was inhibited by ADMA and SDMA with half maximal inhibitory concentration (IC50) values of 758 and 789 μM, respectively [71]. L-homoarginine inhibited L-arginine transport with an IC50 of 1320 μM [73]. These IC50 values suggest that L-arginine transport is unlikely to be affected in a clinically important manner by physiological concentrations of ADMA, SDMA, and L-homoarginine.
The lack of CAT1 in homozygous knockout SLc7a1 mice was found to be lethal [109]. Loss of function of L-arginine transport of up to 70% by small interfering RNA (siRNA)-mediated knockdown of SLc7a1 resulted in delayed conceptus growth and abnormal function compared to the wild type [110]. These data suggest that at least in mice CAT1 is crucial during early development.
A study by Kakoki et al. linked rat CAT1 to hypertension [111]. They observed that infusion with an antisense oligonucleotide against CAT1 lead to a reduction of CAT1 expression in the renal medulla that was associated with decreased NO levels and development of hypertension in rats. In line with this Yang et al. observed an association of a single nucleotide polymorphism (SNP) ss52051869 (g.29514470G > A) in the 3′UTR of human SLC7A1 with predisposition to essential hypertension in the Australian population [112]. Using SLc7a1 transgenic mice the authors showed that overexpression SLc7a1 results in significantly higher NO production and greater sensitivity of endothelial cells to acetylcholine compared with the wild-type mice. Later on, the same authors found that the altered SLC7A1 expression was possibly mediated by disruption of miR-122 (micro RNA 122) binding [113]. Following this, a Finnish cohort study [114] reported another SNP in the SLC7A1 gene (rs41318021; g.29514470G > A). Herein, it was reported that after a 15-year follow-up study, the CT or TT variants of the SNP were associated with a moderately higher predisposition to elevated diastolic blood pressure than the CC genotype.
However, in two independent clinical studies assessing whole blood transcriptome-wide gene expression, SLC7A1 expression was found to be elevated in patients with atrial fibrillation [115,116]. In contrast to the SNP studies, associating SNPs related to impaired expression with hypertension, the elevation of expression in atrial fibrillation could also reflect a response to atrial fibrillation, rather than being its cause. Furthermore, several studies showed that SLC7A1 expression can be modulated by amino acid supplementation, drugs or hormones, possibly mediating (adverse) drug effects.
Among others, cyclosporine A (CsA) was found to inhibit the cellular uptake of L-arginine by modulating CAT1 protein levels in human umbilical vein endothelial cells (HUVECs), which may result in impaired NO production and endothelial dysfunction [117]. The authors showed that CAT1 abundance was significantly reduced following a 48h incubation with CsA. These findings may explain some of the adverse cardiovascular effects of CsA, such as hypertension. Similarly, a study in HUVECs showed that progesterone inhibits arginine transport by modulating CAT1 expression via both protein kinase C-alpha (PKC α) and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation [118]. In contrast, CAT1 transport activity was found to be stimulated by estradiol through modulation of constitutive signaling transduction pathways involving extracellular signal-regulated kinase. These data may explain some of the observed effects of female sex hormones on the NO-mediated endothelial function [118].
Moreover, it has been reported that continued release of thyroid hormones may modulate mRNA expression of CAT1 through various mechanisms (e.g., integrin inhibition, genetic αv subunit down-regulation, or by phosphatidyl-inositol-3 kinase, mitogen-activated protein kinases, or intracellular calcium signaling inhibitors) which modulated L-arginine transport and results in altered endothelial production of NO and vascular tone. Thus, alterations of this mechanism may involve in some of the cardiovascular abnormalities in thyroid disorders [119]. Inflammatory mediators (e.g., tumor necrosis factor-α) (TNF-α) have been found to play a role in CAT1-mediated influx and efflux of L-arginine transport in endothelial cells [120].
Given the prominent role of CAT1 in cellular uptake and exchange of L-arginine and its emerging links to vascular function and hypertension (Table 4), CAT1 deserves further investigation not only as a target for adverse drug effects affecting CAT1-mediated uptake of L-arginine, but also as potential target to modulate the uptake and distribution of L-arginine and its derivatives in cardiovascular disease.

Table 4.
Transporters of L-arginine and its derivatives involved in the pathophysiology of the cardiovascular system.
SLC7A2 (CAT2A and CAT2B)
The human cationic amino acid transporter 2 (CAT2) was initially cloned from human intestine cDNA library [121]. The SLC7A2 gene is located on chromosome 8p22. Alternative splicing of the SLC7A2 gene leads to the expression of CAT2A and CAT2B comprising 657 and 658 amino acids, respectively. They vary in 19 residues in the region of transmembrane helices 8 and 9 [72]. Both transporters facilitate the cellular uptake and exchange of cationic amino acids, but differ in their tissue distribution and transport characteristics.
In contrast to CAT1, CAT2A and CAT2B have a more restricted expression pattern [61]. CAT2A is predominantly expressed in the liver, with additional, but much lower, expression in pancreas, cardiomyocytes, skeletal and vascular smooth muscle, and in cardiac endothelial cells [66]. From studies investigating cationic amino acid transport in mouse liver evidence emerged for the existence of a low-affinity but high-capacity transporter [122]. In line with this, HEK cells overexpressing human CAT2A show a matching low-affinity, high-capacity transport pattern (Table 3) [123].
The transport mechanisms of these CAT transporters have been studied in various experimental models.
The affinity of human CAT2A for L-arginine and ADMA is relatively low, with KM values of approximately 3000 μM and 4000 μM, respectively (Table 5) [72,123]. CAT2A transport is not saturated by L-homoarginine, whilst no direct data are available for the transport of SDMA by this transporter [73]. Of note, significant CAT2A-mediated uptake of both ADMA and L-arginine was detected only at high ADMA and L-arginine concentrations and not at physiological levels [123].

Table 5.
Characteristics of plasma membrane transporters that accept L-arginine and its derivatives as substrates.
The cationic amino acid transporter 2 (CAT2B) is expressed as an inducible protein with a still low (KM value of 952 μM) but slightly higher affinity for L-arginine than the one of CAT2A [123], despite its close similarity. A previous study from our laboratory using human embryonic kidney (HEK) cells overexpressing CAT2B demonstrated that CAT2B transported L-arginine and ADMA with KM values of 952 and ≈4021 μM, respectively, and VMax values of 15,300 and ≈14,300 nmol × mg protein−1 × min−1, respectively [123]. Similar to CAT2A, the CAT2B-mediated transport of SDMA has not been investigated yet.
The liver plays an important role, not only in L-arginine uptake and metabolism by arginases, but also in the absorption and metabolism of ADMA and SDMA from the circulation [29,127,128,129,130]. Altered, i.e., mostly elevated, plasma concentrations of L-arginine [131] and its methylated derivatives [132,133,134] have been reported in liver diseases, i.e., impaired liver function. So far, there is no direct evidence if high L-arginine or ADMA concentrations may affect CAT2A expression. Nevertheless, it can be assumed that in all of these cases, the ability of CAT2A to facilitate quantitative uptake of L-arginine and ADMA at high concentrations may play a role. As CAT2 mRNA is abundantly expressed in the liver, the low affinity but high capacity of CAT2A for L-arginine and related substrates [123] enables this organ to remove excess (post prandial) cationic amino acids from the blood without significantly competing with the uptake of theses substrates by transport systems of other tissues at lower concentrations [122].
While CAT1 is ubiquitously expressed [68], its extracellular L-arginine uptake is decreasing once intracellular cationic amino acids are depleted [67] (Table 4). Therefore, cells with higher arginine consumption (e.g., macrophages) [135] may induce CAT2B expression to provide immediate transport of L-arginine to meet functional needs. In line with this, a number of studies reported that CAT2B function is a potential target to modulate immunity [135,136,137,138]. Several mediators and drugs may stimulate the expression of CAT2B leading to increased cellular L-arginine uptake. Among others, rapamycin stimulates CAT2B mRNA and protein expression in HUVECs resulting in increased L-arginine transport [139]. Several inflammatory cytokines (e.g., interleukin 4 and interleukin 10, interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) have been reported to stimulate expression and L-arginine transport in macrophages [140]. Moreover, TNF-α and supernatants of both Pseudomonas aeruginosa and Staphylococcus aureus also stimulate mRNA and protein expression of CAT2B and L-arginine influx in human corneal epithelial cells [141], suggesting that under inflammatory conditions, cells with inducible CAT2B may increase L-arginine availability particularly for iNOS.
In addition, CAT2 is also responsible for the transport of L-arginine in myeloid-derived suppressor cells (MDSC) and is an important modulator of the MDSC suppressive function of T cell response [the original publication does not distinguish CAT2A and CAT2B]. In mouse models of prostate-specific inflammation and cancer, CAT2 deficient-MDSC demonstrated significantly reduced capacity for modulating T cell responses due to diminished L-arginine supply for NO production [148]. In these models, it was shown that lack of CAT2 leads to enhanced antitumor activity. Furthermore, lack of CAT2 in murine models showed protective effects against hyperoxia-induced acute lung injury [149]. Considering the cardiopulmonary pathophysiological impacts of hyperoxia [150], lack of CAT2 may play a vital role in such conditions.
In a recent cardiovascular health study, SLC7A2 mRNA expression was found to be downregulated by the micro RNA hsa-miR-545 in myocardial infarction [142]. Moreover, in a GWAS study in Japanese population, a SNP (rs56335308; g.7561951: G > A) in the SLC7A2 gene was found to be associated with plasma L-arginine concentrations [151]. However, it is still unclear whether these genetic variants directly modify the risk of CVD. In 2019, a first clinical case of mutations in the SLC7A2 gene was reported [152]. Genetic analysis identified two loss-of-function mutations in the SLC7A2 gene, which includes maternal allele deletion at c.874delA (p.Ile292Leufs*2) and one large genomic rearrangement encompassing exons 3 and 4 in the paternal allele. However, no data is available whether this loss of function is affecting either CAT2A or CAT2B or both proteins. The pathobiochemical phenotype was characterized by an increasing concentration of cationic amino acids in plasma and urine. However, it is yet unknown if this patient will develop cardiovascular diseases in a long-term follow-up, though the findings of this study suggest a possible reduction in iNOS activity.
SLC7A3 (CAT3)
The human cationic amino acid transporter 3 (CAT3), encoded by the SLC7A3 gene, was initially cloned from human peripheral tissues [153]. This gene is located on X-chromosome (Xq13.1) encoding a 619-amino acid protein [153]. In rodents, CAT3 is considered as a brain-specific transporter. However, in humans the highest expression of mRNA has been reported in the thymus, followed by expression in the brain, uterus, mammary gland, testis, stomach, and ovary [153]. Moreover, CAT3 is highly expressed during fetal development, and predicted to be involved in embryogenesis and fetal development [154].
CAT3 can be kinetically distinguished from other members of the CAT family by a different affinity to L-lysine [154]. CAT3 mediates the sodium-independent transport of cationic amino acids with KM values for L-arginine ranging from 40 to 120 µM (mouse CAT3) and 450 µM (human CAT3) [154,155]. It yet remains to be elucidated whether this transporter also transports ADMA, SDMA, and L-homoarginine.
SLC7A3 appears to be highly sensitive to genetic variations in humans, as documented by the low frequency of detrimental variants in available databases [156]. However, a rare genetic variant of the SLC7A3 has been described in male individuals, and it has been suggested that in association with other genetic factors SLC7A3 variants possibly contribute to the etiology of autism spectrum disorder (ASD) in male subjects [156]. These associations apart possible vascular effects have not been described yet.
SLC7A6 (y+LAT2) and SLC7A7 (y+LAT1)
The SLC7A6 (located on chromosome 16q22.1–16q22.2) and SLC7A7 genes (located on chromosome 14q11.2) are members of the SLC7 family. SLC7A6 (encoding for the y+LAT2 protein) and SLC7A7 (encoding for the y+LAT1 protein) are similar in substrate selectivity and function. Both function as heterodimers, requiring the interaction between a “transporter” subunit and a chaperone subunit. The chaperone is responsible for the trafficking of the transporter to the plasma membrane [56]. The SLC3A2 gene encoding the chaperone 4F2hc, consists of 529 amino acids and is also member of the SLC family and acts as chaperone for both of y+LAT2 and y+LAT1.
The y+L transporters are high-affinity transport systems for both cationic and neutral amino acids, with a unique feature of differential Na+-dependence: the influx of neutral amino acids is coupled to Na+ influx, whereas the efflux of cationic amino acids is independent to Na+ [157]. Both y+LAT1 and y+LAT2 are expressed in the intestine and in the kidney, where they localize to the basolateral membrane of absorptive epithelial cells (for a review see [158]). Several other tissues (e.g., brain, testis, parotid, heart, lung, and liver), were also reported to express these transporters [159,160,161,162]. Moreover, SLC7A7 expression is also found in leucocytes, placenta, and lung [55].
These heterodimeric SLC transporters play a critical role in the release of absorbed cationic amino acids from the intestinal lumen and from kidney epithelial cells into the blood. They act as an exchanger by exporting cationic amino acids (e.g., L-arginine and its derivatives, L-ornithine, and L-lysine) into the blood in exchange for large neutral amino acids (e.g., glutamine, leucine) [55]. It has been shown that the system y+L facilitates L-arginine transport in human umbilical vein endothelial cells (HUVECs) with a KM value of 42 μM [120]. However, these transport studies did not clearly distinguish between y+LAT1 and y+LAT2. Similar affinities were observed for L-arginine transport by human y+LAT1 (in monocyte-derived macrophages) and human y+LAT2 (in fibroblast) with KM values of 182 and 145 μM, respectively (Table 2). To present, the transport of other L-arginine derivatives by both of y+LAT1 and y+LAT2 have not been studied.
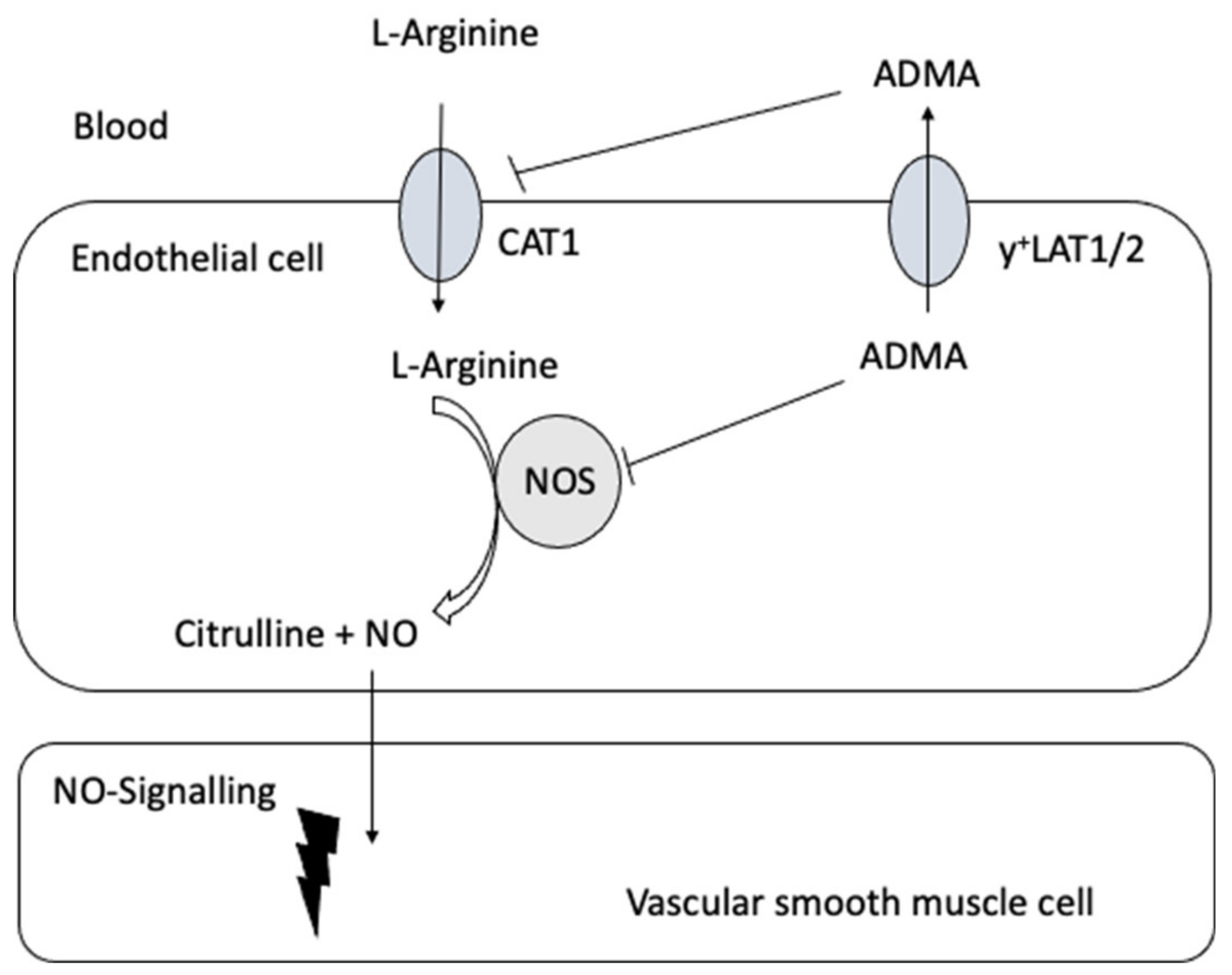
Closs et al. [143] provided the first clinical evidence linking impaired expression of y+LAT1 to coronary and endothelial dysfunction resulting from an impaired cellular ADMA efflux and therefore intracellular ADMA accumulation (Figure 3 and Table 4). Oral L-arginine supplementation improved coronary and peripheral endothelial function and increased plasma ADMA concentrations. The latter was attributed to increased release of the elevated intracellular ADMA levels by alternative exchange transport systems (such as CATs) driven by the cationic amino acid L-arginine. These results suggest that under physiological conditions, y+LAT1 activity is fundamental in reducing excessive intracellular ADMA levels for the maintenance of endothelial function. Moreover, these data indicate that at least some forms of vascular disease involving endothelial dysfunction may be amendable to therapeutic interventions directed at defective cellular transport.
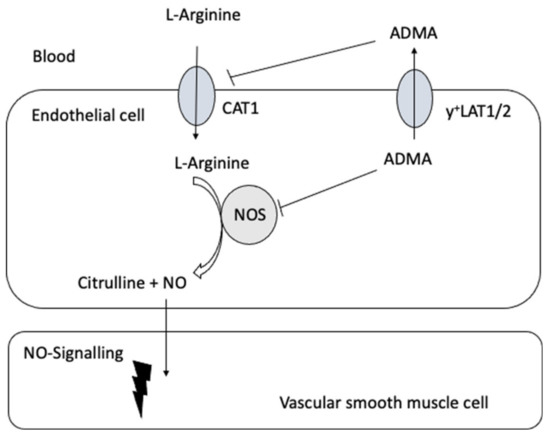
Figure 3.
A schematic diagram of the possible interrelation of the transport of L-arginine and its derivatives and nitric oxide (NO) signaling in the vasculature.
It has been shown that inflammation is associated with an increased L-arginine transport mediated by y+LAT1. A number of proinflammatory cytokines, notably IFN-γ, have been described to exerts significant effects on the expression of y+LAT1 [163] but not y+LAT2, indicating that SLC7A7 is sensitive to IFN-γ. Furthermore, recent in vitro studies using astrocytes and rat brain endothelial cells demonstrated that y+LAT2 may have protective effects against hyperammonemic conditions in the brain [164]. In this study, ammonia-mediated up-regulation of y+LAT2 diminished NO synthesis by increasing intracellular ADMA and SDMA depletion without any significant changes in L-arginine concentrations. These in vitro findings suggest that y+LAT2 may play a role in the pathogenesis of hyperammonemic encephalopathies.
Several studies indicate that genetic variations in the SLC7A7 gene may result in a genetic predisposition for CVD [106,165]. In 2001, Kamada et al. demonstrated the effect of an L-arginine deficiency due to lysinuric protein intolerance (LPI) on vascular endothelial function in humans. In the reported case, the authors identified two mutations (5.3-kbp Alu–mediated deletion and IVS3+1 G→A) in the SLC7A7 gene, which lead to a possible loss or a very low y+LAT1 activity [106]. Further analysis of these mutations revealed an insufficiency of brachial arterial dilatation after L-arginine infusion, low plasma L-arginine concentrations and impaired NO generation in endothelial cells. The authors also showed that following L-arginine supplementation, both NO generation and vascular endothelial function in the patient were normalized. Moreover, Kayanoki et al. report a marked endothelial dysfunction related to L-arginine deficiency in a patient later diagnosed with LPI [165]. In support of this, several studies reported that cardiovascular events are more prevalent in LPI patients [106,166,167], indicating that the SLC7A7 gene is likely to be relevant in the pathogenesis of CVD.
SLC7A9-SLC3A1 (b0,+AT-rBAT)
The heterodimeric b0,+AT-rBAT transporter encoded by the SLC7A9 and SLC3A1 genes, is also known as “cystine transporter”, based on its association with cystinuria detailed further below [168]. This protein was named based on its transport process; b0,+AT (“b” for broad; lowercase letter represent sodium independent active transport, and the superscript “0 and +” designating the net charge on the neutral and cationic amino acid substrates, respectively. Whereas, “r” in rBAT is referred to “related to BAT (BAT = b0,+ amino acid transport)”. The primary transport activity is mediated by b0,+AT, while rBAT shows no transport activity and acts as a chaperone for directing b0,+AT to the correct plasma membrane. The rBAT (SLc3a1 gene) was first isolated by expression cloning in Xenopus oocytes using both rat and rabbit kidney cDNAs [169,170,171]. The human SLC3A1 gene was mapped to chromosome 2p21 [172]. The SLC7A9 gene is located on chromosome 19, region q13.11 [173]. The b0,+AT-rBAT is a high affinity transporter for cationic amino acids and has a low affinity for neutral amino acids with different mode of Na+ dependency: the uptake of cationic amino acids is independent to Na+, whereas the efflux of neutral amino acids is Na+-dependent [174]. In humans, b0,+AT-rBAT proteins are predominantly expressed in the luminal membrane of kidney tubular cells of the kidney and enterocytes [175].
This protein complex facilitates the electrogenic transport of extracellular cystine and cationic amino acids for intracellular neutral amino acids [176]. On the intracellular site, the affinity for neutral amino acids is in the millimolar range [177]. In contrast, on the extracellular site, b0,+AT-rBAT has a high affinity for cationic amino acids and cystine (micromolar range) [177], which suggests a key role of this transporter in the renal reabsorption of cystine and cationic amino acids from the urine (Figure 2). It has been reported that the half-saturation constant for human L-arginine measured in different expression system was within the range of 100 μM to 180 μM [178,179,180] (Table 5). Whether b0,+AT-rBAT also transports ADMA, SDMA, and L-homoarginine remains to be elucidated.
Inactivating mutations in the human SLC7A9 and SLC3A1 genes result in the partial failure of cationic amino acid reabsorption, clinically impressing as cystinuria. Cystinuric patients excrete increased amount of cystine and cationic amino acids with their urine [181]. Urinary excretion of L-arginine and L-homoarginine is increased by factors of one hundred-fold and ten-fold, respectively in patients with cystinuria while the plasma concentrations of L-arginine and L-homoarginine are significantly reduced compared to healthy subjects [100]. The heteromeric protein transporter b0,+AT-rBAT was found to be encoded by rather frequently mutated genes.
So far, approximately 160 mutations have been identified in the SLC3A1 gene and 116 mutations have been identified in the SLC7A9 gene [182]. Most mutations identified in cystinuria are missense mutations leading to the change of a single amino acid in the protein. The effect of these mutations can range from having no effect on protein function to loss of function [183]. It is estimated that approximately 99% of cationic amino acids are reabsorbed at the apical membrane of proximal kidney tubules [96,97], indicating that b0,+AT-rBAT mediated transport may have a considerable leverage on the homeostasis of cationic amino acids.
In a retrospective cohort study, which involved 442 patients with cystinuria an increased prevalence rate of hypertension with age was observed [144]. Another research group in a specialist cystinuria centre reported an overall prevalence of hypertension in subjects with cystinuria of 50.8% (61 out of 120 patients were hypertensive), with prevalence in males almost two-fold that of females (62.1% vs. 37.0%) [145]. When compared with the prevalence of hypertension in the normal healthy population, the prevalence of hypertension in cystinuric patients were 2-fold in men and 1.3-fold in women.
It was also shown that hypertension was associated with the stage of chronic kidney diseases (CKD); among the patients with normal renal function: 34.1% (10/29) were hypertensive, 50% (34/68) in the CKD stage 2, and 81% (17/21) of those with CKD stage 3 [145]. Taken together, these data support a role of SLC7A9 and SLC3A1 mutations in the genesis of hypertension. However, little is known regarding the precise underlying mechanisms. It would be of interest to evaluate plasma levels of L-arginine and its derivatives in relation to the long-term clinical outcome in patients with cystinuria.
2.1.3. SLCO/SLC21 Family
The SLC21/SLCO family was initially named SLC21; however, the nomenclature of its members has been subjected to changes in 2004 based on phylogenetic relationships, and the family was designated to SLC21/SLCO, the solute carrier family of the organic anion transport polypeptides (OATPs) [239]. The SLC21/SLCO family represents a large group of proteins transporting a variety of substrates, including anionic, neutral and cationic compounds. Eleven different OATPs have been identified in humans of which, so far, (only) one member, the organic anion transport polypeptide 4C1 (OATP4C1), was found to be capable of transporting L-arginine and its derivatives [212].
SLCO4C1 (OATP4C1)
The SLCO4C1 gene encoding human OATP4C1 was mapped to chromosome 5q21.2 and encodes a protein with 724 amino acid residues. OATP4C1 is expressed in the basolateral membrane of the kidney proximal tubules [240]. This transporter facilitates Na+-independent uptake of a large variety of compounds and it is regarded as the first step in the vectorial transport (from blood across proximal tubule cells into urine) of these compounds (including uremic toxins) [241].
OATP4C1 mediates the transport of structurally diverse compounds including endogenous substances as such thyroid hormones (e.g., T3 and T4), cyclic AMP (cAMP) as well as drugs such as cardiac glycosides (e.g., digoxin and ouabain), methotrexate, and the dipeptidyl peptidase-4 inhibitor sitagliptin [240,242]. Although several members of the SLC21/SLCO family have been identified in detail, the functional characteristics of OATP4C1 still warrant further research. A recent study using stably-transfected HEK cells overexpressing human OATP4C1 showed that OATP4C1 can mediate the uptake as well as the efflux of L-arginine (uptake KM of 48.1 μM) ADMA (uptake KM value of 232.1 μM) and homoarginine (uptake KM value of 49.9 µM) [212] (Table 5), suggesting that OATP4C1 may be involved in the excretion of uremic toxins in renal failure. Uptake of L-homoarginine could be inhibited by ADMA and SDMA with IC50 values of 117 and 54 μM, respectively. These inhibitory concentrations are well above the physiological range. A follow up study using double-transfected MDCK-OATP4C1-p-gp cells simultaneously expressing basolaterally localized OATP4C1 and luminally localized export pump P-glycoprotein demonstrated vectorial transport of L-arginine, L-homoarginine, and ADMA suggesting, that also this ABC-transporter may be important for their renal homeostasis of these compounds [185].
Altered OATP4C1 expression has been linked to CVD events [146,243] (Table 4) and endometrial cancer tissues [209], and genetic variants of the SLCO4C1 gene were also found to be associated with obesity [244]. Increased expression of human OATP4C1 in a transgenic rat kidney model leads to an increased excretion of uremic toxins and reduced hypertension and cardiomegaly [146,243]. In addition, there is emerging evidence that genetic polymorphisms of SLCO4C1 gene in patients with cardiac insufficiency contribute to renal clearance of digoxin. Based on a population pharmacokinetic model, SLCO4C1 SNPs (rs3114660 and rs3114661) were associated with a significantly altered the renal clearance of digoxin [210].
2.1.4. SLC22 Family
There are currently six subfamilies of SLC22 transporters, which have been predominantly studied as drug transporters, so far: (1) The organic cation transporter (OCT), (2) The organic cation/carnitine transporter (OCTN), (3) OCT/OCTN-related, (4) Organic anion transporter (OAT), (5) The OAT-like, and (6) The OAT-related [245]. Little is known about their role in the cellular uptake and release of endogenously formed molecules. As detailed below, recent studies suggest a contributing role of some of these transporters in the cellular handling of L-arginine and its derivatives [123].
SLC22A2 (OCT2)
The organic cation transporter 2 (OCT2) is encoded by the SLC22A2 gene located on chromosome 6q26. The protein is composed of 555 amino acid residues. This transporter is a low-affinity, high-capacity carrier that mediates the sodium-independent transport of cationic compounds [246]. OCT2 is strongly expressed in the proximal tubules of the kidney, located in the basolateral membrane in cells of the S2 and S3 region. Less abundant expression of OCT2 is also observed in the brain, the inner ear, the small intestine, and the lung [247,248,249,250].
Previously identified OCT2 substrates include tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP+), the oral antidiabetic drug metformin, and cimetidine [251]. OCT2 facilitates the first step in the renal elimination of compounds and may control the intracellular concentrations of monoamine neurotransmitters in the brain [252]. It has been reported that the human OCT2-mediated L-arginine uptake may not be physiologically saturable (the predicted KM value is higher than 10,000 μM).
In addition, the Michaelis constant of ADMA for human OCT2 (KM: 900 ± 100 μM) [123] greatly exceeds physiological (about 0.4–0.9 μM) and even pathophysiological concentrations of ADMA (up to 8 μM in certain diseases) [253] in human plasma, indicating that human OCT2 could function as an ADMA transporter without saturation. This low-affinity transport of ADMA by OCT2 may contribute to the renal elimination of ADMA from circulation. No data regarding SDMA and L-homoarginine as substrates are available, so far.
Numerous polymorphisms in the SLC22A2 gene have been identified [254]. However, most of these genetic variants have only been associated with drugs disposition and efficacy. In a recent meta-analysis using an integrated genome-transcriptome approach, SLC22A2 gene expression was found to be significantly increased in pre-hypertensive groups [147] (Table 4). So far, the impact of SLC22A2 SNPs on the renal clearance of uremic toxins such as ADMA has never been studied but, deserves to be further investigated.
2.1.5. SLC25 Family
The SLC25 family is the largest SLC family in humans and composed of at least 53 family members. The SLC25 transporters mediate the transport of diverse substrates (e.g., various metabolites, nucleotides, and coenzymes) across the inner membrane of mitochondria. So far, approximately one-third of the putative mitochondrial transporters are “orphans”, with no known substrates [255]. However, until now, at least three transporters SLC25A2, SLC25A15, and SLC25A29 are known to transport L-arginine and/or its derivatives [220,221].
SLC25A2 (ORNT2), SLC25A15 (ORNT1), and SLC25A29 (ORNT3)
The SLC25A2 and SLC25A15 genes encode mitochondrial ornithine transporters 2 (ORNT2) and ornithine transporters 1 (ORNT1), respectively. ORNT1 was the first of the two isoforms to be identified and characterized [256], followed by the highly homologues ORNT2 [257]. Both proteins share 88% amino acid identity. The SLC25A2 gene is located on chromosome 5q31.3 13q14.11 and the SLC25A15 gene on chromosome 13q14.11 The product of the SLC25A29 gene was firstly described as carnitine/acylcarnitine transporter-like (CACL) protein by Sekoguchi et al. [258]. Later, Camacho and Rioseco-Camacho classified this gene as ornithine transporter isoform 3 (ORNT3) because its overexpression retrieves the defect in ornithine uptake in cultured fibroblasts from patients with the hyperornithinemiahyperammonemia-homocitrullinuria (HHH) syndrome [223]. However, further study presented direct evidence that ORNT3 is a mitochondrial transporter responsible for the transport of basic amino acids in preference to L-arginine and L-lysine [227]. The SLC25A29 gene, encoding ORNT3 is located on chromosome 14q32.2.
ORNT2 was observed at the inner mitochondrial membrane in cells of several organs such as liver, testis, spleen, lung, pancreas, small intestine, and brain [259]. ORNT1 is expressed in mitochondria of liver, pancreas, lung, testis, small intestine, spleen, brain, and the heart [259]. Interestingly, SLC25A2mRNA is expressed in the human kidney whereas SLC25A15 could not be detected there [256]. ORNT3 is expressed in mitochondria located in the liver, in heart, in skeletal muscle, and in the brain/inner mitochondrial membrane [227].
These three mitochondrial transporters have at least several transport properties in common. They transport L-ornithine, L-lysine, L-arginine, and L-citrulline by homo or heteroexchange and unidirectionally, and they are inhibited by the same inhibitors [125]. Regarding L-arginine and its derivatives, human ORNT1 was reported to facilitate the transport of only L-arginine, so far (a KM value for L-arginine is 1500 μM) [220]. In contrast to ORNT1, ORNT2 has a wider substrate specificity. ORNT2 also facilitates the transport D-isomers of ornithine, lysine, arginine, and citrulline and as well as histidine, L-homoarginine, and ADMA [260], which all can be metabolized in the mitochondria. The affinity of ORNT2 for L-arginine is approximately 710 μM [220,221]. ORNT2 facilitates both, the exchange of substrates as well as unidirectional transport. The reported initial rates of the L-arginine homoexchange and L-arginine/ADMA exchanges were 290 and 240 pmol × mg protein−1 × min−1, respectively; while the initial rates of the L-arginine as uniport was 50 pmol × mg protein−1 × min−1. Notably, the transport activities of the L-arginine/ADMA exchanges were similar to those of the arginine homoexchanges. The ORNT2-mediated ADMA transport was within the same range as the transport of the prototypic ORNT2 substrates known, so far, with a KM value of 370 μM; and was highly specific, since symmetric dimethylarginine (SDMA), the structural isomer of ADMA, was not transported at all. Moreover, ADMA inhibited ORNT2-mediated transport of L-lysine with an inhibition constant of 380 μM, whereas SDMA, although not transported, showed some inhibition of ORNT2 transport activity [221].
Transport of ADMA could be a byproduct of mitochondrial L-arginine uptake. However, given that there are enzymes in the mitochondrial space which can metabolize ADMA, SDMA and L-homoarginine, it is likely that the transport of ADMA across the mitochondrial membrane could have different functions. The influx of ADMA into mitochondria could enable its degradation by mitochondrial alanine-glyoxylate aminotransferase 2 (AGXT2). Conversely, in cells lacking AGXT2 expression, ORNTs could mediate the mitochondrial efflux of ADMA produced by mitochondrial proteolysis for cytosolic degradation by DDAHs or for further efflux from the cell by transporters in the plasma membrane to allow degradation in other tissues [221]. L-homoarginine has also been reported to be transported by ORNT2, however, its transport kinetics remain to be defined [26,94,220].
The main function of the ornithine transporter 3 (ORNT3) is thought to facilitate the transport of L-arginine, L-lysine, L-histidine into mitochondria for mitochondrial protein synthesis. This transporter also recognizes L-homoarginine as a substrate [227]. The affinity of ORNT3 for L-arginine is moderate, with a KM value of 420 μM [220,225]. Porcelli et al. demonstrated that ORNT3-reconstituted liposomes take up external L-arginine more efficiently in the presence of internal L-arginine, L-lysine, and L-homoarginine compared to ornithine, histidine, or carnitine, or compared to the absence of any internal substrate [227]. This transporter mediates the transport of L-arginine in two different ways, as exchange (homo or heteroexchange) or as uniport. The transport affinity for L-arginine as exchange (homo/heteroexchange) or as uniport is rather similar, with KM values of 420 μM and 470 μM, respectively. By contrast, the average VMax of L-arginine uptake measured as homo/heteroexchange is 3.1-fold higher than that derived from uniport, i.e., 237 pmol × mg protein−1 × min−1. This transporter therefore can mediate either L-arginine uptake or release. It was shown that L-homoarginine can competitively inhibit L-arginine exchange with an inhibitory constant (Ki) value of 450 μM, however no clear data is available regarding the transport kinetics of L-homoarginine [227]. To present, no data have been reported related to the ORNT3-mediated transport of ADMA and SDMA.
Until now, no genetic variations in the human SLC25A29 gene have been identified that link it to any diseases. In contrast, genetic variations in the SLC25A2 and SLC25A15 genes have been shown to be responsible for the hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome [256]. However, this rare syndrome is beyond the scope of this review. In the context of CVD, no studies have been done related to the role of mitochondrial transporters, in this respect it is of interest, that SLC25A2 likely plays an important role in regulating ADMA at the mitochondrial level. As recent studies highlight the role of mitochondrial AGXT2 in the metabolism of ADMA and L-homoarginine [26,94], the mitochondrial transport of these substrates certainly warrants further investigation.
2.1.6. SLC38 Family
The SLC38 (System A and System N sodium-coupled neutral amino acid transporter) family is composed of 11 members, encoded by the genes SLC38A1-11 [125]. The members of this group are characterized as sodium-coupled neutral amino acid transporters (SNATs) [261] with glutamine as preferred substrate throughout the family. So far three human SNATs are known to transport L-arginine [229,230,232]: SLC38A4 (SNAT4), SLC38A7 (SNAT7), and SLC38A8 (SNAT8).
SLC38A4 (SNAT4), SLC38A7 (SNAT7), and SLC38A8 (SNAT8)
The SLC38A4 gene, encoding for the SNAT4 protein, is located on chromosome 12q13 and the protein consists of 547 amino acids. This transporter is related to the members of the glutamine transporter family. SNAT4 is expressed in the liver [229] and in the placenta [262]. The SLC38A7 gene (encoding SNAT7 protein) and SLC38A8 gene (encoding SNAT8 protein) are clustered together in the chromosomal region 16q21 and q23.3, respectively. Both, SNAT7 and SNAT8, are expressed predominantly in the central nervous system, almost exclusively in neurons [230,232]. Both of these transporters mediate Na+-dependent influx and efflux of small neutral amino acids [125].
In humans, the sodium-coupled neutral amino acid transporter 4 (SNAT4) facilitates the transport of neutral amino acids and K-(methylamino) isobutyric acid (MeAIB) in a Na+-dependent manner, and the transport of cationic amino acids in a Na+-independent manner [125]. This transporter has a higher affinity for cationic amino acids (KM: 300 μM for L-arginine) compared to neutral amino acids (KM: 1600 μM for L-glycine), which is similar to that of y+LATs [229]. The transport of other L-arginine derivatives (ADMA, SDMA, and L-homoarginine) by this transporter has not been reported yet.
SNAT7 and SNAT8 are sodium coupled (system N) transporters of the SLC38 family and known as lysosomal glutamine transporters. In a Xenopus oocyte model overexpression of SNAT7 was associated with a significant increase of L-arginine uptake [230]. Similarly, SNAT8 also showed a high preference for L-glutamine, L-alanine, L-histidine, L-aspartate and L-arginine [232]. Similar to SNAT4, the transport of ADMA, SDMA, and L-homoarginine by these two transporters remain to be investigated.
While CAT2A is a low-affinity transporter with a KM value for L-arginine within the millimolar range, SNAT4 has an about 10-times higher affinity for L-arginine (KM: 300 μM) [229]. Since SNAT4 is expressed abundantly in the liver, it is assumed that this transporter largely contributes to the cellular uptake of L-arginine into hepatocytes under physiological conditions [229]. However, if SNAT4 contributes relevantly to the total uptake of L-arginine into the liver remains doubtful for at least one reason: the liver expresses highly levels of arginase 1 which catalyzes the hydrolysis of L-arginine to urea and L-ornithine [263]. Thus, the functionally significant presence of a high-affinity L-arginine transporter (in addition to the abundant expression of CAT2) would increase the amount of L-arginine taken up by the liver and limit the availability of arginine for other pathways including NOS signaling.
Among the three transporters mentioned here, the consequences of gene deletion are known only for murine SLc38a4 [226]. The paternal knockout of SLc38a4 shows an intrauterine growth retardation. In humans a SNP of SLC38A8 (c.1234G>A[p.Gly412Arg]) was found to be associated with foveal hypoplasia [231]. It remains to be elucidated whether these three members of SLC38 family also have a function in the cardiovascular system related to L-arginine and its derivatives.
2.1.7. The SLC47 Family
The SLC47 family is composed of two human members MATE1 (SLC47A1) and MATE2-K (SLC47A2). At present, L-arginine and ADMA transport has been characterized only for MATE1 126].
SLC47A1 (MATE1)
The multidrug and toxin extrusion protein 1 (MATE1) is a 570 amino acid integral membrane protein encoded by the SLC47A1 gene located on chromosome 17p11.2. In humans, transcripts of MATE1 are ubiquitously expressed in the body, including kidney, liver, brain, colon, lung, placenta, and skeletal muscle [264]. However, at protein level, MATE1 could be detected in kidney and liver (located in the luminal membrane of proximal tubular cells and the canalicular membranes of hepatocytes) [265,266]. Functionally, MATE1 is an electroneutral, sodium-independent, and pH-dependent proton antiporter for organic compounds [267].
MATE1 mediates the bidirectional transport of organic cations and depends on a proton gradient. Thus, this protein may function as an uptake or efflux transporter depending on the orientation of the proton gradient [268,269]. MATE1 recognizes various organic cations, some non-charged compounds, and some zwitterions as substrates [266]. Various endogenous compounds, such as creatinine, thiamine, guanidine, and estrone-3-sulfate, were identified as human MATE1 substrates [266].
A recent study showed that MATE1 can mediate the uptake of L-arginine and ADMA [123]. The uptake of L-arginine and ADMA is pH-dependent, with uptake of L-arginine and ADMA markedly increased following alkalinization of the extracellular medium [123]. In this study, MATE1 facilitated the cellular uptake of L-arginine and ADMA with a rather low activity and uptake ratios at pH 7.3 of only 1.2 and 1.1, respectively (Table 5). It is still of interest that ADMA can be recycled to L-citrulline in the kidney [270]. To date, the MATE1-mediated transport properties of SDMA and L-homoarginine are unknown.
The uptake (with subsequent metabolism) or excretion of ADMA by MATE1 may be rather unspecific, attributable to its broad substrate spectrum, it may, however, under certain circumstances contribute to the renal elimination of ADMA. With respect to CVD its role in the renal excretion of xenobiotics, including drugs may be of more relevance, though.
2.2. The Adenosine Triphosphate (ATP)-Binding Cassette (ABC) Superfamily
The ATP-binding cassette (ABC) superfamily consists of two types of proteins, the cytosolic ABC-ATPases and the ABC transporters. The major part of the ABC transporters is located at the plasma membrane and actively transports a wide array of substrates with hydrolysis of adenosine triphosphate (ATP) as the driving force. There are currently at least 50 known human ABC transporters classified into seven groups from ABCA to ABCG. The vast majority of these transporters are expressed as exporters facilitating the efflux of a variety of substrates. The ABCB subfamily consists of 11 members. Its most prominent member is likely ABCB1 it is also known as multidrug resistance protein or permeability-glycoprotein (P-glycoprotein, P-gp). To date, only ABCB1 has been shown to mediate the transport of L-arginine and its derivatives [213].
ABCB1/MDR1 (P-glycoprotein)
P-glycoprotein (P-gp) is an active membrane bound efflux transport protein pump. It was first identified in the membranes of cancer cells, where it contributed to the phenomenon of multidrug resistance [271]. The ABCB1 gene is located on chromosome 7q21.12 and encodes 1280 amino acid residues. Besides being prominently expressed in the apical membrane of epithelial cells in the intestine and in the kidney proximal tubules [272], ABCB1 is also expressed in the biliary membrane of hepatocytes and numerous other tissues (apical surface of brain capillary endothelial cells, adrenal gland, pancreatic ductile, and placental trophoblast, and in the arterioles and capillaries of the left ventricular myocardium) [273]. A signature role of this adenosine triphosphate (ATP)-driven transport protein is its ability to recognize a variety of structurally diverse molecules ranging in size from 100 to 4000 daltons (Da) (molecular mass) [274].
P-gp is mostly known as an efflux pump protein for a wide range of drugs: [275,276]. The function of P-glycoprotein (P-gp) can be categorized according to its anatomical localization (Figure 2): P-gp limits xenobiotic (i.e., drug) absorption in intestinal tissues; P-gp protects sensitive organs and tissues (i.e., the brain) by extruding its substrates from the cells or tissues into the circulation. Furthermore, P-gp also facilitates clearance of drugs and metabolites into bile and urine [277]. In the kidney, P-gp is specifically expressed at the side of the tubular apical membrane, limiting the systemic exposure to xenobiotics by extruding the molecules from the epithelial cells to the luminal space [278].
P-gp contribution to the transport of L-arginine and SDMA has never been studied before, yet, it was only recently that ADMA and L-homoarginine had been associated with P-gp [213]. This ATP-driven transporter paired with the SLCO4C1 transporter was reported to facilitate the efflux of ADMA and L-homoarginine through a transcellular transport (from the basolateral to the apical compartment) with transport ratios of 2.0- and 3.4-fold, respectively.
As with other transport proteins with broad spectrum of substrates, transport of L-homoarginine could simply be attributed to unspecific activity. However, given its broad expression it is likely that P-gp contributes to the transport and regulation of both L-arginine derivatives. Apart from its role in the plasma disposition of aldosterone [279,280,281], no obvious associations between P-gp activity towards biomarkers and cardiovascular disease have been reported yet.
3. Conclusions
L-arginine is the key substrate for NO-mediated signaling in the cardiovascular system and the plasma concentrations of its derivatives L-homoarginine, ADMA and SDMA are independently associated with cardiovascular outcomes and mortality. However, despite the substantial evidence linking these substances more or less directly to cardiovascular physiology and outcomes their functional interrelation remains only partially understood, leaving the question unsettled where the “risk marker” ends and rather the “risk factor” begins. Here a better understating of the transport mechanisms governing the cellular uptake and exchange of these substances may provide some necessary insights, which are currently lacking.
From the evidence discussed, the identification of distinct transport proteins that facilitate the transport of L-arginine and its derivatives will likely improve our understanding on the effects of L-arginine and its derivatives in maintaining vascular health. The identification of the truly relevant transport systems, with respect to cardiovascular disease is far from easy, though. Especially as the number of transporter proteins found to transport L-arginine and its derivatives is continuously growing, with about 18 human transporters identified so far.
In principle, lower KM values indicate higher affinities and it is tempting to directly compare KM values reported in different publications. However, before doing so, methodological issues should be carefully considered. Difficulties in determining the contributions of an individual transport protein often arise from the redundancy of transport systems for amino acids and related compounds in most tissues, with multiple alternative transport proteins for a single substrate available. For this reason, often only “apparent” rather than absolute transport activities can be provided for living cells, because it is practically impossible to block endogenous transport activity except for that of interest. From a biological perspective, these redundancies may also have advantages, as they would render the system less sensitive to impairment of an individual transport protein. The term “apparent” also indicates the KM and VMax values should be taken with a grain of salt, especially when comparing KM values of different transporters which are often obtained in different experimental settings.
Given the importance of substrates like L-arginine for cellular function, these redundancies are not surprising. However, redundant (i.e., “background “) transport by different transport systems puts some limits the characterization of the contribution of individual transport proteins in all but the most artificial experimental settings, which in turn come with other limitations. Furthermore, molecules with divergent pathophysiological properties such as L-arginine and ADMA share major transport systems, making it difficult to gauge the net in vivo impact of an individual transport system in CVD.
Despite all these limitations, the largely “apparent” transport data obtained from cell lines overexpressing transporters of interest already allow substantial insights regarding the putative functional role of the transporters with regard to the respective substrates and possible impact on cardiovascular physiology. Based on the data available so far, members of the CAT and LAT family such as CAT1 and y+LAT1/2 can be considered as major players, especially with respect to endothelial function and cardiovascular disease. Furthermore, case report data indicate that a y+LAT1 related transport deficiency may be corrected by L-arginine supplementation treatment [143].
Studies of human diseases, animal models, and cultured cells have demonstrated that there are at least two different functions through which L-arginine and its derivatives transporters can be related to CVD. Firstly, by providing adequate L-arginine for NO production. Secondly, by contributing to the disposition and homeostasis of L-arginine and its derivatives. It will be a key issue for future research in this area to identify the transport proteins and/or molecular mechanisms mediating differential transport of the chemically very similar substrates (L-arginine and L-homoarginine as compared to ADMA and SDMA). The relative changes in the concentration ratio of L-arginine and SDMA between plasma and urine are highly suggestive of a transport system that allows the selective depletion of L-arginine and L-homoarginine from the urine while SDMA is excreted into the urine. These chemically similar substances may actually turn out to be useful tools for the dissection of differential transport mechanisms. However, given the much higher plasma and tissue concentration of L-arginine, any apparently differential transport of L-homoarginine or ADMA and SDMA may simply reflect differences in substrate concentration than evolved differences in substrate specificity of the respective transporter.
Finally, drugs may affect the transport of L-arginine and its derivatives, possibly contributing to adverse as well as beneficial effects in CVD. Alternatively, selective inhibition of (re-)absorption of ADMA and SDMA at sites like the small intestine or the kidney could enhance their elimination. Conversely, selective inhibition of L-arginine and L-homoarginine uptake by tissues primarily involved in their degradation and elimination could increase their availability for tissues where they support protective functions, such as endothelial function. It remains to be shown, however, that the latter two mechanisms could also work in humans.
Author Contributions
Conceptualization, S.B. and R.M.; writing—original draft preparation, S.B.; writing—review and editing, S.B., R.M., R.N.R., and J.K.; visualization, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. rer. biol. hum.” S.B. was supported by LPDP (Indonesia Endowment Fund for Education, Ministry of Finance, Republic of Indonesia).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 10 June 2020).
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nat. Cell Biol. 1988, 333, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Does ADMA Cause Endothelial Dysfunction? Arter. Thromb. Vasc. Biol. 2000, 20, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Creager, M.A. Arginine and Endothelial and Vascular Health. J. Nutr. 2004, 134, 2880S–2887S. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.W.; Mattson, D.L. Role of l-arginine in nitric oxide production in health and hypertension. Clin. Exp. Pharmacol. Physiol. 2009, 36, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.; Zeiher, A.; Meinzer, K.; Just, H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet 1991, 338, 1546–1550. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Qin, L.-Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral l-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M.; Kuller, L.H. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef]
- Drover, J.W.; Dhaliwal, R.; Weitzel, L.; Wischmeyer, M.P.E.; Ochoa, J.B.; Heyland, D.K. Perioperative Use of Arginine-Supplemented Diets: A Systematic Review of the Evidence. J. Am. Coll. Surg. 2011, 212, 385–399. [Google Scholar] [CrossRef]
- Piatti, P.; Monti, L.D.; Valsecchi, G.; Magni, F.; Setola, E.; Marchesi, F.; Galli-Kienle, M.; Pozza, G.; Alberti, K.G.M. Long-Term Oral L-Arginine Administration Improves Peripheral and Hepatic Insulin Sensitivity in Type 2 Diabetic Patients. Diabetes Care 2001, 24, 875–880. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Closs, E.I.; Scheld, J.S.; Sharafi, M.; Förstermann, U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: Role of cationic amino acid transporters. Mol. Pharmacol. 2000, 57, 68–74. [Google Scholar] [PubMed]
- Simon, A.; Plies, L.; Habermeier, A.; Martiné, U.; Reining, M.; Closs, E.I. Role of Neutral Amino Acid Transport and Protein Breakdown for Substrate Supply of Nitric Oxide Synthase in Human Endothelial Cells. Circ. Res. 2003, 93, 813–820. [Google Scholar] [CrossRef]
- Shin, S.; Mohan, S.; Fung, H.-L. Intracellular l-arginine concentration does not determine NO production in endothelial cells: Implications on the “l-arginine paradox”. Biochem. Biophys. Res. Commun. 2011, 414, 660–663. [Google Scholar] [CrossRef]
- Atzler, D.; Schwedhelm, E.; Choe, C. L-Homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Böger, M.R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Endogenous Dimethylarginine as an Inhibitor of Nitric Oxide Synthesis. J. Cardiovasc. Pharmacol. 1992, 20, S60–S62. [Google Scholar] [CrossRef]
- Goonasekera, C.D.; Rees, D.D.; Woolard, P.; Frend, A.; Shah, V.; Dillon, M.J. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J. Hypertens. 1997, 15, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Itoh, S.; Kimoto, M.; Kohno, K.; Tamai, O.; Wada, Y.; Yasukawa, H.; Iwami, G.; Okuda, S.; Imaizumi, T. Asymmetrical Dimethylarginine, an Endogenous Nitric Oxide Synthase Inhibitor, in Experimental Hypertension. Hypertension 1997, 29, 242–247. [Google Scholar] [CrossRef]
- Fliser, D.; Kielstein, J.T.; Haller, H.; Bode-Böger, S.M. Asymmetric dimethylarginine: A cardiovascular risk factor in renal disease? Kidney Int. 2003, 63, S37–S40. [Google Scholar] [CrossRef][Green Version]
- Ronden, R.A.; Houben, A.J.H.M.; Teerlink, T.; Bakker, J.A.; Bierau, J.; Stehouwer, C.D.A.; De Leeuw, P.W.; Kroon, A.A. Reduced renal plasma clearance does not explain increased plasma asymmetric dimethylarginine in hypertensive subjects with mild to moderate renal insufficiency. Am. J. Physiol. Physiol. 2012, 303, F149–F156. [Google Scholar] [CrossRef]
- Colonna, V.D.G.; Bianchi, M.; Pascale, V.; Ferrario, P.; Morelli, F.; Pascale, W.; Tomasoni, L.; Turiel, M. Asymmetric dimethylarginine (ADMA): An endogenous inhibitor of nitric oxide synthase and a novel cardiovascular risk molecule. Med Sci. Monit. 2009, 15, 91–101. [Google Scholar]
- Leiper, J.; Nandi, M.; Torondel, B.; Murray-Rust, J.; Malaki, M.; O’Hara, B.; Rossiter, S.; Anthony, S.; Madhani, M.; Selwood, D.; et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat. Med. 2007, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.; Impraim, B.; Simmel, S.; Bode-Böger, S.M.; Tsikas, D.; Frölich, J.C.; Hoeper, M.M.; Haller, H.; Fliser, D. Cardiovascular Effects of Systemic Nitric Oxide Synthase Inhibition With Asymmetrical Dimethylarginine in Humans. Circulation 2004, 109, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric Dimethylarginine Causes Hypertension and Cardiac Dysfunction in Humans and Is Actively Metabolized by Dimethylarginine Dimethylaminohydrolase. Arter. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Murry, D.J.; Vaulman, S.F.; Stevens, J.W.; Lentz, S.R. Human Alanine-Glyoxylate Aminotransferase 2 Lowers Asymmetric Dimethylarginine and Protects from Inhibition of Nitric Oxide Production. J. Biol. Chem. 2009, 285, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Martens-Lobenhoffer, J.; Brilloff, S.; Burdin, D.V.; Jarzebska, N.; Demyanov, A.V.; Hohenstein, B.; Weiss, N.; Bode-Böger, S.M. Acetylation of asymmetric and symmetric dimethylarginine: An undercharacterized pathway of metabolism of endogenous methylarginines. Nephrol. Dial. Transplant. 2015, 31, 57–63. [Google Scholar] [CrossRef][Green Version]
- Kielstein, J.T.; Fliser, D.; Veldink, H. Asymmetric Dimethylarginine and Symmetric Dimethylarginine: Axis of Evil or Useful Alliance? Semin. Dial. 2009, 22, 346–350. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Teerlink, T.; Siroen, M.P.; van Lambalgen, A.A.; Rauwerda, J.A.; van Leeuwen, P.A. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin. Nutr. 2003, 22, 17–22. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Choe, C.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine Levels Are Regulated by l -Arginine:Glycine Amidinotransferase and Affect Stroke Outcome. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grübler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.Ó.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef]
- Moali, C.; Boucher, J.-L.; Sari, M.-A.; Stuehr, A.D.J.; Mansuy, D. Substrate Specificity of NO Synthases: Detailed Comparison ofl-Arginine, Homo-l-arginine, TheirNω-Hydroxy Derivatives, andNω-Hydroxynor-l-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Hrabák, A.; Bajor, T.; Temesi, A. Comparison of Substrate and Inhibitor Specificity of Arginase and Nitricm Oxide (NO) Synthase for Arginine Analogs and Related Compounds in Murine and Rat Macrophages. Biochem. Biophys. Res. Commun. 1994, 198, 206–212. [Google Scholar] [CrossRef]
- Brӧer, S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006, 48, 559–567. [Google Scholar] [CrossRef]
- Nelson, N. The Family of Na+/Cl− Neurotransmitter Transporters. J. Neurochem. 2002, 71, 1785–1803. [Google Scholar] [CrossRef]
- Kekuda, R.; Torres-Zamorano, V.; Fei, Y.-J.; Prasad, P.D.; Li, H.W.; Mader, L.D.; Leibach, F.H.; Ganapathy, V. Molecular and functional characterization of intestinal Na(+)-dependent neutral amino acid transporter B0. Am. J. Physiol. Content 1997, 272, G1463–G1472. [Google Scholar] [CrossRef]
- Sloan, J.L.; Mager, S. Cloning and Functional Expression of a Human Na+and Cl−-dependent Neutral and Cationic Amino Acid Transporter B0+. J. Biol. Chem. 1999, 274, 23740–23745. [Google Scholar] [CrossRef]
- Kekuda, R.; Prasad, P.D.; Fei, Y.-J.; Torres-Zamorano, V.; Sinha, S.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Cloning of the Sodium-dependent, Broad-scope, Neutral Amino Acid Transporter Bofrom a Human Placental Choriocarcinoma Cell Line. J. Biol. Chem. 1996, 271, 18657–18661. [Google Scholar] [CrossRef]
- Sun, L.; Rommens, J.M.; Corvol, H.; Li, W.; Li, X.; A Chiang, T.; Lin, F.; Dorfman, R.; Busson, P.-F.; Parekh, R.V.; et al. Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat. Genet. 2012, 44, 562–569. [Google Scholar] [CrossRef]
- Doyle, F.A.; McGivan, J.D. The bovine renal epithelial cell line NBL-1 expresses a broad specificity Na+-dependent neutral amino acid transport system (System B°) similar to that in bovine renal brush border membrane vesicles. Biochim. Biophys. Acta Biomembr. 1992, 1104, 55–62. [Google Scholar] [CrossRef]
- Uchiyama, T.; Fujita, T.; Gukasyan, H.J.; Kim, K.-J.; Borok, Z.; Crandall, E.D.; Lee, V.H.L. Functional characterization and cloning of amino acid transporter B0,+ (ATB0,+) in primary cultured rat pneumocytes. J. Cell. Physiol. 2007, 214, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.; Estévez, R.; Bertran, J.; Zorzano, A. Molecular Biology of Mammalian Plasma Membrane Amino Acid Transporters. Physiol. Rev. 1998, 78, 969–1054. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Ganapathy, M.E.; Leibach, F.H. Intestinal transport of peptides and amino acids. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2000; Volume 50, pp. 379–412. [Google Scholar]
- Suviolahti, E.; Oksanen, L.J.; Öhman, M.; Cantor, R.M.; Ridderstråle, M.; Tuomi, T.; Kaprio, J.; Rissanen, A.; Mustajoki, P.; Jousilahti, P.; et al. The SLC6A14 gene shows evidence of association with obesity. J. Clin. Investig. 2003, 112, 1762–1772. [Google Scholar] [CrossRef]
- Gupta, N.; Miyauchi, S.; Martindale, R.G.; Herdman, A.V.; Podolsky, R.; Miyake, K.; Mager, S.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1741, 215–223. [Google Scholar] [CrossRef]
- Gupta, N.; Prasad, P.D.; Ghamande, S.; Moore-Martin, P.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Mager, S.; Ganapathy, M.E.; Ganapathy, V. Up-regulation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol. Oncol. 2006, 100, 8–13. [Google Scholar] [CrossRef]
- Yanai, H.; Ben-Shachar, S.; Baram, L.; Elad, H.; Gitstein, G.; Brazowski, E.; Tulchinsky, H.; Pasmanik-Chor, M.; Dotan, I. Gene expression alterations in ulcerative colitis patients after restorative proctocolectomy extend to the small bowel proximal to the pouch. Gut 2015, 64, 756–764. [Google Scholar] [CrossRef]
- Karunakaran, S.; Umapathy, N.S.; Thangaraju, M.; Hatanaka, T.; Itagaki, S.; Munn, D.H.; Prasad, P.D.; Ganapathy, V. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 2008, 414, 343–355. [Google Scholar] [CrossRef]
- Corvol, H.; Blackman, S.M.; Boëlle, P.-Y.; Gallins, P.J.; Pace, R.G.; Stonebraker, J.R.; Accurso, F.J.; Clement, A.; Collaco, J.M.; Dang, H.; et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015, 6, 8382. [Google Scholar] [CrossRef]
- Di Paola, M.; Park, A.J.; Ahmadi, S.; Roach, E.J.; Wu, Y.-S.; Struder-Kypke, M.; Lam, J.S.; Bear, C.E.; Khursigara, C.M. SLC6A14Is a Genetic Modifier of Cystic Fibrosis That RegulatesPseudomonas aeruginosaAttachment to Human Bronchial Epithelial Cells. mBio 2017, 8, e02073-17. [Google Scholar] [CrossRef]
- Gutiérrez, M.L.; Corchete-Sánchez, L.; Teodósio, C.; Sarasquete, M.-E.; Abad, M.D.M.; Iglesias, M.; Esteban, C.; Sayagués, J.M.; Orfao, A.; Muñoz-Bellvís, L. Identification and characterization of the gene expression profiles for protein coding and non-coding RNAs of pancreatic ductal adenocarcinomas. Oncotarget 2015, 6, 19070–19086. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Bhutia, Y.D.; Ramachandran, S.; Gnanaprakasam, J.P.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. Deletion of the amino acid transporter Slc6a14 suppresses tumour growth in spontaneous mouse models of breast cancer. Biochem. J. 2015, 469, 17–23. [Google Scholar] [CrossRef]
- Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflügers Archiv. Eur. J. Physiol. 2004, 447, 532–542. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- White, M.F.; Christensen, H.N. Cationic amino acid transport into cultured animal cells. II. Transport system barely perceptible in ordinary hepatocytes, but active in hepatoma cell lines. J. Biol. Chem. 1982, 257, 4450–4457. [Google Scholar] [PubMed]
- I Closs, E.; Gräf, P. Cationic amino acid transporters (CATs). Targets for the manipulation of NO-synthase activity? Pharm. Biotechnol. 1999, 12, 229–249. [Google Scholar]
- Kavanaugh, M.P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry 1993, 32, 5781–5785. [Google Scholar] [CrossRef]
- Albritton, L.M.; Kim, J.W.; Tseng, L.; Cunningham, J.M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J. Virol. 1993, 67, 2091–2096. [Google Scholar] [CrossRef]
- Closs, E.I.; Boissel, J.-P.; Habermeier, A.; Rotmann, A. Structure and Function of Cationic Amino Acid Transporters (CATs). J. Membr. Biol. 2006, 213, 67–77. [Google Scholar] [CrossRef]
- Devés, R.; Boyd, C.A.R. Transporters for Cationic Amino Acids in Animal Cells: Discovery, Structure, and Function. Physiol. Rev. 1998, 78, 487–545. [Google Scholar] [CrossRef]
- Hatzoglou, M.; Fernandez, J.; Yaman, I.; Closs, E. Regulation of catonic amino acid transport: The story of the CAT-1 transporter. Annu. Rev. Nutr. 2004, 24, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Closs, E.I.; Albritton, L.M.; Cunningham, J.M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nat. Cell Biol. 1991, 352, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Vanoaica, L.; Behera, A.; Camargo, S.M.; Forster, I.C.; Verrey, F. Real-time functional characterization of cationic amino acid transporters using a new FRET sensor. Pflügers Archiv Eur. J. Physiol. 2015, 468, 563–572. [Google Scholar] [CrossRef]
- Closs, E.I. CATs, a family of three distinct mammalian cationic amino acid transporters. Amino Acids 1996, 11, 193–208. [Google Scholar] [PubMed]
- Closs, E.I.; Simon, A.; Vékony, N.; Rotmann, A. Plasma Membrane Transporters for Arginine. J. Nutr. 2004, 134, 2752S–2759S. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.; Pacitti, A.J.; Souba, W.W. Characterization of L-arginine transport by pulmonary artery endothelial cells. Am. J. Physiol. Cell. Mol. Physiol. 1993, 264, L351–L356. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.K.; I Zharikov, S.; Block, E.R.; Kilberg, M.S. A Caveolar Complex between the Cationic Amino Acid Transporter 1 and Endothelial Nitric-oxide Synthase May Explain the “Arginine Paradox”. J. Biol. Chem. 1997, 272, 31213–31216. [Google Scholar] [CrossRef]
- Closs, E.I.; Basha, F.Z.; Habermeier, A.; Förstermann, U. Interference ofL-Arginine Analogues withL-Arginine Transport Mediated by the y+Carrier hCAT-2B. Nitric Oxide 1997, 1, 65–73. [Google Scholar] [CrossRef]
- Strobel, J.; Mieth, M.; Endreß, B.; Auge, D.; König, J.; Fromm, M.F.; Maas, R. Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1). J. Mol. Cell. Cardiol. 2012, 53, 392–400. [Google Scholar] [CrossRef]
- Closs, E.I.; Gräf, P.; Habermeier, A.; Cunningham, J.M.; Förstermann, U. Human Cationic Amino Acid Transporters hCAT-1, hCAT-2A, and hCAT-2B: Three Related Carriers with Distinct Transport Properties. Biochemistry 1997, 36, 6462–6468. [Google Scholar] [CrossRef]
- Chafai, A.; Fromm, M.F.; König, J.; Maas, R. The prognostic biomarker L-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2A and CAT2B. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lüneburg, N.; Xanthakis, V.; Schwedhelm, E.; Sullivan, L.M.; Maas, R.; Anderssohn, M.; Riederer, U.; Glazer, N.L.; Vasan, R.S.; Böger, R.H. Reference Intervals for Plasma L-Arginine and the L-Arginine:Asymmetric Dimethylarginine Ratio in the Framingham Offspring Cohort. J. Nutr. 2011, 141, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Möller, P.; Alvestrand, A.; Bergström, J.; Fürst, P.; Hellström, K. Electrolytes and Free Amino Acids in Leg Skeletal Muscle of Young and Elderly Women. Gerontology 1983, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Bergström, J.; Eriksson, S.; Fürst, P.; Hellström, K. Effect of Aging on Free Amino Acids and Electrolytes in Leg Skeletal Muscle. Clin. Sci. 1979, 56, 427–432. [Google Scholar] [CrossRef]
- Atzler, D.; Schwedhelm, E.; Nauck, M.; Ittermann, T.; Böger, R.H.; Friedrich, N. Serum reference intervals of homoarginine, ADMA, and SDMA in the Study of Health in Pomerania. Clin. Chem. Lab. Med. 2014, 52, 1835–1842. [Google Scholar] [CrossRef]
- Atzler, D.; Mieth, M.; Maas, R.; Böger, R.H.; Schwedhelm, E. Stable isotope dilution assay for liquid chromatography-tandem mass spectrometric determination of l-homoarginine in human plasma. J. Chromatogr. B 2011, 879, 2294–2298. [Google Scholar] [CrossRef]
- Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Kruszelnicka, O.; Rycaj, J.; Chyrchel, B.; Surdacki, A.; Bode-Böger, S.M. Opposite Associations of Plasma Homoarginine and Ornithine with Arginine in Healthy Children and Adolescents. Int. J. Mol. Sci. 2013, 14, 21819–21832. [Google Scholar] [CrossRef]
- Kayacelebi, A.A.; Beckmann, B.; Gutzki, F.-M.; Jordan, J.; Tsikas, D. GC–MS and GC–MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids 2014, 46, 2205–2217. [Google Scholar] [CrossRef]
- May, M.; Kayacelebi, A.A.; Batkai, S.; Jordan, J.; Tsikas, D.; Engeli, S. Plasma and tissue homoarginine concentrations in healthy and obese humans. Amino Acids 2015, 47, 1847–1852. [Google Scholar] [CrossRef]
- Böger, R.H. The pharmacodynamics of L-arginine. Altern. Ther. Heal. Med. 2014, 20, 48–54. [Google Scholar] [CrossRef]
- Wu, G.; Morris, J.S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Ajami, A.; Branch, S.; Chapman, T.; Yu, Y.-M.; Burke, J.; Young, V. Plasma arginine kinetics in adult man: Response to an arginine-free diet. Metabolism 1994, 43, 114–122. [Google Scholar] [CrossRef]
- Anthony, S.; Leiper, J.; Vallance, P. Endogenous production of nitric oxide synthase inhibitors. Vasc. Med. 2005, 10, 3–9. [Google Scholar] [CrossRef]
- Päivä, H.; Lehtimäki, T.; Laakso, J.; Ruokonen, I.; Tervonen, R.; Metso, S.; Nikkilä, M.; Wuolijoki, E.; Laaksonen, R. Dietary composition as a determinant of plasma asymmetric dimethylarginine in subjects with mild hypercholesterolemia. Metabolism 2004, 53, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Che, D.; Qin, G.; Farouk, M.H.; Hailong, J.; Rui, H. Novel Biosynthesis, Metabolism and Physiological Functions of L-Homoarginine. Curr. Protein Pept. Sci. 2018, 20, 184–193. [Google Scholar] [CrossRef]
- Kayacelebi, A.A.; Langen, J.; Weigt-Usinger, K.; Chobanyan-Jürgens, K.; Mariotti, F.; Schneider, J.Y.; Rothmann, S.; Frölich, J.C.; Atzler, R.; Choe, C.; et al. Biosynthesis of homoarginine (hArg) and asymmetric dimethylarginine (ADMA) from acutely and chronically administered free l-arginine in humans. Amino Acids 2015, 47, 1893–1908. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine Metabolism Revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef]
- Xuan, C.; Xu, L.-Q.; Tian, Q.-W.; Li, H.; Wang, Q.; He, G.-W.; Lun, L.-M. Dimethylarginine Dimethylaminohydrolase 2 (DDAH 2) Gene Polymorphism, Asymmetric Dimethylarginine (ADMA) Concentrations, and Risk of Coronary Artery Disease: A Case-Control Study. Sci. Rep. 2016, 6, 33934. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Akazawa, S. Isolation and identification of N-G,N-G- and N-G,N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J. Biol. Chem. 1970, 245, 5751–5758. [Google Scholar]
- Seppälä, I.; Kleber, M.E.; Lyytikäinen, L.-P.; Hernesniemi, J.; Mäkelä, K.-M.; Oksala, N.; Laaksonen, R.; Pilz, S.; Tomaschitz, A.; Silbernagel, G.; et al. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. Eur. Heart J. 2013, 35, 524–531. [Google Scholar] [CrossRef]
- Ogawa, T.; Kimoto, M.; Watanabe, H.; Sasaoka, K. Metabolism of NG,NG- and NG,NG-dimethylarginine in rats. Arch. Biochem. Biophys. 1987, 252, 526–537. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Oppici, E.; Martens-Lobenhoffer, J.; Jarzebska, N.; Brilloff, S.; Burdin, D.; Demyanov, A.; Kolouschek, A.; Leiper, J.; Maas, R.; et al. A Novel Pathway for Metabolism of the Cardiovascular Risk Factor Homoarginine by alanine:glyoxylate aminotransferase 2. Sci. Rep. 2016, 6, 35277. [Google Scholar] [CrossRef] [PubMed]
- Bollenbach, A.; Cordts, K.; Hanff, E.; Atzler, R.; Choe, C.-U.; Schwedhelm, E.; Tsikas, D. Evidence by GC-MS that lysine is an arginase-catalyzed metabolite of homoarginine in vitro and in vivo in humans. Anal. Biochem. 2019, 577, 59–66. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Renal Arginine Metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef]
- Tizianello, A.; De Ferrari, G.; Garibotto, G.; Gurreri, G.; Robaudo, C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J. Clin. Investig. 1980, 65, 1162–1173. [Google Scholar] [CrossRef]
- Van De Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; DeJong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Doolan, P.D.; Harper, H.A.; Hutchin, M.E.; Shreeve, W.W. Renal Clearance of Eighteen Individual Amino Acids in Human Subjects 1. J. Clin. Investig. 1955, 34, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.D.; Cameron, J.S. Homoarginine in Cystinuria. Clin. Sci. Mol. Med. 1974, 46, 173–182. [Google Scholar] [CrossRef]
- Torremans, A.; Marescau, B.; Vanholder, R.; De Smet, R.; Billiouw, J.-M.; De Deyn, P.P. The low nanomolar levels of N G -monomethylarginine in serum and urine of patients with chronic renal insufficiency are not significantly different from control levels. Amino Acids 2003, 24, 375–381. [Google Scholar] [CrossRef]
- Frenay, A.-R.S.; Kayacelebi, A.A.; Beckmann, B.; Soedamah-Muhtu, S.S.; De Borst, M.H.; Berg, E.V.D.; Van Goor, H.; Bakker, S.J.L.; Tsikas, D. High urinary homoarginine excretion is associated with low rates of all-cause mortality and graft failure in renal transplant recipients. Amino Acids 2015, 47, 1827–1836. [Google Scholar] [CrossRef]
- Jacobi, J.; Tsao, P.S. Asymmetrical dimethylarginine in renal disease: Limits of variation or variation limits? A systematic review. Am. J. Nephrol. 2007, 28, 224–237. [Google Scholar] [CrossRef]
- Drechsler, C.; Kollerits, B.; Meinitzer, A.; März, W.; Ritz, E.; König, P.; Neyer, U.; Pilz, S.; Wanner, C.; Kronenberg, F.; et al. Homoarginine and Progression of Chronic Kidney Disease: Results from the Mild to Moderate Kidney Disease Study. PLoS ONE 2013, 8, e63560. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished Global Arginine Bioavailability and Increased Arginine Catabolism as Metabolic Profile of Increased Cardiovascular Risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Nagaretani, H.; Tamura, S.; Ohama, T.; Maruyama, T.; Hiraoka, H.; Yamashita, S.; Yamada, A.; Kiso, S.; Inui, Y.; et al. Vascular endothelial dysfunction resulting from l-arginine deficiency in a patient with lysinuric protein intolerance. J. Clin. Investig. 2001, 108, 717–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.-U.; Böger, R.H.; De Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and Cardiovascular Outcome in the Population-Based Dallas Heart Study. Arter. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Teerlink, T.; Scheffer, P.G.; Meinitzer, A.; Rutters, F.; Tomaschitz, A.; Drechsler, C.; Kienreich, K.; Nijpels, G.; Stehouwer, C.D.A.; et al. Homoarginine and mortality in an older population: The Hoorn study. Eur. J. Clin. Investig. 2014, 44, 200–208. [Google Scholar] [CrossRef]
- Perkins, C.P.; Mar, V.; Shutter, J.R.; Del Castillo, J.; Danilenko, D.M.; Medlock, E.S.; Ponting, I.L.; Graham, M.; Stark, K.L.; Zuo, Y.; et al. Anemia and perinatal death result from loss of the murine ecotropic retrovirus receptor mCAT-1. Genes Dev. 1997, 11, 914–925. [Google Scholar] [CrossRef]
- Wang, X.; Frank, J.W.; Little, D.R.; Dunlap, K.A.; Satterfield, M.C.; Burghardt, R.C.; Hansen, T.R.; Wu, G.; Bazer, F.W. Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J. 2014, 28, 2852–2863. [Google Scholar] [CrossRef]
- Kakoki, M.; Wang, W.; Mattson, D.L. Cationic Amino Acid Transport in the Renal Medulla and Blood Pressure Regulation. Hypertension 2002, 39, 287–292. [Google Scholar] [CrossRef]
- Yang, Z.; Venardos, K.; Jones, E.; Morris, B.J.; Chin-Dusting, J.; Kaye, D.M. Identification of a Novel Polymorphism in the 3′UTR of thel-Arginine Transporter GeneSLC7A1. Circulation 2007, 115, 1269–1274. [Google Scholar] [CrossRef]
- Yang, Z.; Kaye, D.M. Mechanistic insights into the link between a polymorphism of the 3′UTR of theSLC7A1gene and hypertension. Hum. Mutat. 2008, 30, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Määttä, K.; Kunnas, T.; Nikkari, S.T. Contribution of SLC7A1 genetic variant to hypertension, the TAMRISK study. BMC Med Genet. 2013, 14, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yin, X.; Lunetta, K.L.; Dupuis, J.; McManus, D.D.; Lubitz, S.A.; Magnani, J.W.; Joehanes, R.; Munson, P.J.; Larson, M.G.; et al. Whole Blood Gene Expression and Atrial Fibrillation: The Framingham Heart Study. PLoS ONE 2014, 9, e96794. [Google Scholar] [CrossRef] [PubMed]
- Thériault, S.; Whitlock, R.; Raman, K.; Vincent, J.; Yusuf, S.; Paré, G. Gene Expression Profiles for the Identification of Prevalent Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, 006057. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Shashar, M.; Bahry, D.; Pri-Paz, Y.; Ben Tur, O.; Levi, S.; Chernichovski, T.; Chernin, G.; Schwartz, I.F. Cyclosporine Attenuates Arginine Transport, in Human Endothelial Cells, through Modulation of Cationic Amino Acid Transporter-1. Am. J. Nephrol. 2013, 37, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Bentur, O.S.; Schwartz, D.; Chernichovski, T.; Ingbir, M.; Weinstein, T.; Chernin, G.; Schwartz, I.F. Estradiol augments while progesterone inhibits arginine transport in human endothelial cells through modulation of cationic amino acid transporter-1. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R421–R427. [Google Scholar] [CrossRef]
- Toral, M.; Jimenez, R.; Montoro-Molina, S.; Romero, M.; Wangensteen, R.; Duarte, J.; Vargas, F. Thyroid hormones stimulate L-arginine transport in human endothelial cells. J. Endocrinol. 2018, 239, 49–62. [Google Scholar] [CrossRef]
- Sala, R.; Rotoli, B.M.; Colla, E.; Visigalli, R.; Parolari, A.; Bussolati, O.; Gazzola, G.C.; Dall’Asta, V. Two-way arginine transport in human endothelial cells: TNF-α stimulation is restricted to system y+. Am. J. Physiol. Physiol. 2002, 282, C134–C143. [Google Scholar] [CrossRef]
- Hoshide, R.; Ikeda, Y.; Karashima, S.; Matsuura, T.; Komaki, S.; Kishino, T.; Niikawa, N.; Endo, F.; Matsuda, I. Molecular Cloning, Tissue Distribution, and Chromosomal Localization of Human Cationic Amino Acid Transporter 2 (HCAT2). Genomics 1996, 38, 174–178. [Google Scholar] [CrossRef]
- I Closs, E.; Albritton, L.M.; Kim, J.W.; Cunningham, J.M. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J. Biol. Chem. 1993, 268, 7538–7544. [Google Scholar]
- Strobel, J.; Müller, F.; Zolk, O.; Endreß, B.; König, J.; Fromm, M.F.; Maas, R. Transport of asymmetric dimethylarginine (ADMA) by cationic amino acid transporter 2 (CAT2), organic cation transporter 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1). Amino Acids 2013, 45, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.; Errasti-Murugarren, E.; Rosell, A. Heteromeric amino acid transporters. In search of the molecular bases of transport cycle mechanisms1. Biochem. Soc. Trans. 2016, 44, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Brӧer, S. The SLC38 family of sodium–amino acid co-transporters. Pflügers Archiv Eur. J. Physiol. 2013, 466, 155–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Sun, K.; Meng, Z.; Chen, L. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J. Mol. Cell Biol. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Carnegie, P.; Fellows, F.; Symington, G. Urinary excretion of methylarginine in human disease. Metabolism 1977, 26, 531–537. [Google Scholar] [CrossRef]
- Siroen, M.P.C.; Van Der Sijp, J.R.M.; Teerlink, T.; Van Schaik, C.; Nijveldt, R.J.; Van Leeuwen, P.A.M. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology 2005, 41, 559–565. [Google Scholar] [CrossRef]
- Inoue, Y.; Bode, B.P.; Beck, D.J.; Li, A.P.; Bland, K.I.; Souba, W.W. Arginine Transport in Human Liver. Ann. Surg. 1993, 218, 350–363. [Google Scholar] [CrossRef]
- Burdin, D.V.; Kolobov, A.A.; Brocker, C.; Soshnev, A.A.; Samusik, N.; Demyanov, A.V.; Brilloff, S.; Jarzebska, N.; Martens-Lobenhoffer, J.; Mieth, M.; et al. Diabetes-linked transcription factor HNF4α regulates metabolism of endogenous methylarginines and β-aminoisobutyric acid by controlling expression of alanine-glyoxylate aminotransferase 2. Sci. Rep. 2016, 6, 35503. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Siroen, M.P.C.; Van Der Hoven, B.; Teerlink, T.; A Prins, H.; Girbes, A.R.J.; Van Leeuwen, P.A.M. High plasma arginine concentrations in critically ill patients suffering from hepatic failure. Eur. J. Clin. Nutr. 2004, 58, 587–593. [Google Scholar] [CrossRef]
- Mookerjee, R.; Dalton, R.N.; Davies, N.; Hodges, S.J.; Turner, C.; Williams, R.; Jalan, R. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transplant. 2007, 13, 400–405. [Google Scholar] [CrossRef]
- Lluch, P.; Torondel, B.; Medina, P.; Segarra, G.; Del Olmo, J.A.; Serra, M.A.; Rodrigo, J.M. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J. Hepatol. 2004, 41, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, R.P.; Malaki, M.; Davies, N.; Hodges, S.J.; Dalton, R.N.; Turner, C.; Sen, S.; Williams, R.; Leiper, J.; Vallance, P.; et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Manner, C.K.; Kleeman, J.; MacLeod, C.L. Sustained Nitric Oxide Production in Macrophages Requires the Arginine Transporter CAT2. J. Biol. Chem. 2001, 276, 15881–15885. [Google Scholar] [CrossRef] [PubMed]
- Yeramian, A.; Martin, L.; Serrat, N.; Arpa, L.; Soler, C.; Bertran, J.; McLeod, C.; Palacín, M.; Modolell, M.; Lloberas, J.; et al. Arginine Transport via Cationic Amino Acid Transporter 2 Plays a Critical Regulatory Role in Classical or Alternative Activation of Macrophages. J. Immunol. 2006, 176, 5918–5924. [Google Scholar] [CrossRef]
- King, N.E.; Rothenberg, M.E.; Zimmermann, N. Arginine in Asthma and Lung Inflammation. J. Nutr. 2004, 134, 2830S–2836S. [Google Scholar] [CrossRef]
- Thompson, R.W.; Pesce, J.T.; Ramalingam, T.; Wilson, M.S.; White, S.; Cheever, A.W.; Ricklefs, S.M.; Porcella, S.F.; Li, L.; Ellies, L.G.; et al. Cationic Amino Acid Transporter-2 Regulates Immunity by Modulating Arginase Activity. PLoS Pathog. 2008, 4, e1000023. [Google Scholar] [CrossRef]
- Visigalli, R.; Barilli, A.; Bussolati, O.; Sala, R.; Gazzola, G.C.; Parolari, A.; Tremoli, E.; Simon, A.; Closs, E.I.; Dall’Asta, V. Rapamycin stimulates arginine influx through CAT2 transporters in human endothelial cells. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1479–1487. [Google Scholar] [CrossRef][Green Version]
- Yeramian, A.; Martin, L.; Arpa, L.; Bertran, J.; Soler, C.; McLeod, C.; Modolell, M.; Palacín, M.; Lloberas, J.; Celada, A. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur. J. Immunol. 2006, 36, 1516–1526. [Google Scholar] [CrossRef]
- Jäger, K.; Bönisch, U.; Risch, M.; Worlitzsch, D.; Paulsen, F.P. Detection and Regulation of Cationic Amino Acid Transporters in Healthy and Diseased Ocular Surface. Investig. Opthalmology Vis. Sci. 2009, 50, 1112–1121. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Wang, Y.; Bai, B.; Yu, T.; Chu, X.-M. The biomarkers of key miRNAs and target genes associated with acute myocardial infarction. PeerJ 2020, 8, e9129. [Google Scholar] [CrossRef]
- Closs, E.I.; Ostad, M.A.; Simon, A.; Warnholtz, A.; Jabs, A.; Habermeier, A.; Daiber, A.; Förstermann, U.; Münzel, T. Impairment of the extrusion transporter for asymmetric dimethyl-l-arginine: A novel mechanism underlying vasospastic angina. Biochem. Biophys. Res. Commun. 2012, 423, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Bataille, P.; Bridoux, F.; Brignon, P.; Choquenet, C.; Cochat, P.; Combe, C.; et al. CKD and Its Risk Factors among Patients with Cystinuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Kum, F.; Wong, K.; Game, D.; Bultitude, M.; Thomas, K. Hypertension and renal impairment in patients with cystinuria: Findings from a specialist cystinuria centre. Urolithiasis 2019, 47, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, T.; Suzuki, T.; Morimoto, R.; Akiyama, Y.; Souma, T.; Shiwaku, H.O.; Takeuchi, Y.; Mishima, E.; Abe, M.; Tanemoto, M.; et al. SLCO4C1 Transporter Eliminates Uremic Toxins and Attenuates Hypertension and Renal Inflammation. J. Am. Soc. Nephrol. 2009, 20, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Campain, A.; Yang, J.Y.H.; Morris, B.J. Meta-Analysis of Genome-Wide Gene Expression Differences in Onset and Maintenance Phases of Genetic Hypertension. Hypertension 2010, 56, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bozkus, C.C.; Elzey, B.D.; Crist, S.A.; Ellies, L.G.; Ratliff, T.L. Expression of Cationic Amino Acid Transporter 2 Is Required for Myeloid-Derived Suppressor Cell-Mediated Control of T Cell Immunity. J. Immunol. 2015, 195, 5237–5250. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Nelin, L.D. Deficiency of cationic amino acid transporter-2 protects mice from hyperoxia-induced lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L598–L607. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Iyer, D.; Rodgers, L.E.; Vanthenapalli, S.; Panguluri, S.K. Impact of hyperoxia on cardiac pathophysiology. J. Cell. Physiol. 2019, 234, 12595–12603. [Google Scholar] [CrossRef]
- Imaizumi, A.; Adachi, Y.; Kawaguchi, T.; Higasa, K.; Tabara, Y.; Sonomura, K.; Sato, T.-A.; Takahashi, M.; Mizukoshi, T.; Yoshida, H.-O.; et al. Genetic basis for plasma amino acid concentrations based on absolute quantification: A genome-wide association study in the Japanese population. Eur. J. Hum. Genet. 2019, 27, 621–630. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Blasco-Alonso, J.; Benito, C.; Rodríguez-García, E.; Andrade, F.; Aldámiz-Echevarría, L.; Muñoz-Hernández, M.C.; Vega, A.I.; Pérez-Cerdá, C.; García-Martín, M.L.; et al. A new metabolic disorder in human cationic amino acid transporter-2 that mimics arginase 1 deficiency in newborn screening. J. Inherit. Metab. Dis. 2019, 42, 407–413. [Google Scholar] [CrossRef]
- Vékony, N.; Wolf, S.; Boissel, J.-P.; Gnauert, K.; Closs, E.I. Human Cationic Amino Acid Transporter hCAT-3 Is Preferentially Expressed in Peripheral Tissues. Biochemistry 2001, 40, 12387–12394. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Sawamura, T.; Masaki, T.; MacLeod, C.L. Increased Cat3-mediated Cationic Amino Acid Transport Functionally Compensates inCat1Knockout Cell Lines. J. Biol. Chem. 1998, 273, 14663–14666. [Google Scholar] [CrossRef]
- Ito, K.; Groudine, M. A New Member of the Cationic Amino Acid Transporter Family Is Preferentially Expressed in Adult Mouse Brain. J. Biol. Chem. 1997, 272, 26780–26786. [Google Scholar] [CrossRef] [PubMed]
- Nava, C.; Rupp, J.; Boissel, J.-P.; Mignot, C.; Rastetter, A.; Amiet, C.; Jacquette, A.; Dupuits, C.; Bouteiller, D.; Keren, B.; et al. Hypomorphic variants of cationic amino acid transporter 3 in males with autism spectrum disorders. Amino Acids 2015, 47, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Meier, C.; Rossier, G.; Kühn, L.C. Glycoprotein-associated amino acid exchangers: Broadening the range of transport specificity. Pflügers Archiv Eur. J. Physiol. 2000, 440, 503–512. [Google Scholar] [CrossRef]
- Brӧer, S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Bröer, A.; Wagner, C.A.; Lang, F.; Brӧer, S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem. J. 2000, 349, 787–795. [Google Scholar] [CrossRef]
- Torrents, D.; Estévez, R.; Pineda, M.; Fernández, E.; Lloberas, J.; Shi, Y.-B.; Zorzano, A.; Palacín, M. Identification and Characterization of a Membrane Protein (y+L Amino Acid Transporter-1) That Associates with 4F2hc to Encode the Amino Acid Transport Activity y+L. J. Biol. Chem. 1998, 273, 32437–32445. [Google Scholar] [CrossRef]
- Boyd, C.; Deves, R.; Laynes, R.; Kudo, Y.; Sebastio, G. Cationic amino acid transport through system y+L in erythrocytes of patients with lysinuric protein intolerance. Pflügers Archiv Eur. J. Physiol. 2000, 439, 513–516. [Google Scholar] [CrossRef]
- Pfeiffer, R.; Rossier, G.; Spindler, B.; Meier, C.; Kühn, L.; Verrey, F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999, 18, 49–57. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Bussolati, O.; Sala, R.; Barilli, A.; Talarico, E.; Gazzola, G.C.; Dall’Asta, V. INFγ stimulates arginine transport through system y+L in human monocytes. FEBS Lett. 2004, 571, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Milewski, K.; Bogacińska-Karaś, M.; Fręśko, I.; Hilgier, W.; Jazwiec, R.; Albrecht, J.; Zielińska, M. Ammonia Reduces Intracellular Asymmetric Dimethylarginine in Cultured Astrocytes Stimulating Its y+LAT2 Carrier-Mediated Loss. Int. J. Mol. Sci. 2017, 18, 2308. [Google Scholar] [CrossRef] [PubMed]
- Kayanoki, Y.; Kawata, S.; Yamasaki, E.; Kiso, S.; Inoue, S.; Tamura, S.; Taniguchi, N.; Matsuzawa, Y. Reduced nitric oxide production by L-arginine deficiency in lysinuric protein intolerance exacerbates intravascular coagulation. Metabolism 1999, 48, 1136–1140. [Google Scholar] [CrossRef]
- Mauhin, W.; Habarou, F.; Gobin, S.; Servais, A.; Brassier, A.; Grisel, C.; Roda, C.; Pinto, G.; Moshous, D.; Ghalim, F.; et al. Update on Lysinuric Protein Intolerance, a Multi-faceted Disease Retrospective cohort analysis from birth to adulthood. Orphanet J. Rare Dis. 2017, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.; Näntö-Salonen, K.; Niinikoski, H.; Jahnukainen, T.; Keskinen, P.; Saha, H.; Kananen, K.; Helanterä, A.; Metso, M.; Linnanvuo, M.; et al. Nephropathy Advancing to End-Stage Renal Disease: A Novel Complication of Lysinuric Protein Intolerance. J. Pediatr. 2007, 150, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Carrascal, M.; Rousaud, F.; Abián, J.; Zorzano, A.; Palacín, M.; Chillarón, J. rBAT-b0,+AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am. J. Physiol. Physiol. 2002, 283, F540–F548. [Google Scholar] [CrossRef]
- Bertran, J.; Werner, A.; Chillarón, J.; Nunes, V.; Biber, J.; Testar, X.; Zorzano, A.; Estivill, X.; Murer, H.; Palacín, M. Expression cloning of a human renal cDNA that induces high affinity transport of L-cystine shared with dibasic amino acids in Xenopus oocytes. J. Biol. Chem. 1993, 268, 14842–14849. [Google Scholar]
- Tate, S.S.; Yan, N.; Udenfriend, S. Expression cloning of a Na(+)-independent neutral amino acid transporter from rat kidney. Proc. Natl. Acad. Sci. USA 1992, 89, 1–5. [Google Scholar] [CrossRef]
- Wells, R.G.; Hediger, M.A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc. Natl. Acad. Sci. USA 1992, 89, 5596–5600. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Rozen, R.; Hediger, M.A.; Goodyer, P.; Eydoux, P. Assignment of the Gene for Cystinuria (SLC3A1) to Human Chromosome 2p21 by Fluorescence in Situ Hybridization. Genomics 1994, 24, 413–414. [Google Scholar] [CrossRef]
- Bisceglia, L.; Calonge, M.J.; Totaro, A.; Feliubadaló, L.; Melchionda, S.; García, J.; Testar, X.; Gallucci, M.; Ponzone, A.; Zelante, L.; et al. Localization, by linkage analysis, of the cystinuria type III gene to chromosome 19q13.1. Am. J. Hum. Genet. 1997, 60, 611–616. [Google Scholar] [PubMed]
- Wagner, C.A.; Lang, F.; Bröer, S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Physiol. 2001, 281, C1077–C1093. [Google Scholar] [CrossRef] [PubMed]
- Feliubadaló, L.; Font, M.; Purroy, J.; Rousaud, F.; Estivill, X.; Nunes, V.; Golomb, E.; Centola, M.; Aksentijevich, I.; Kreiss, Y.; et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat. Genet. 1999, 23, 52–57. [Google Scholar] [CrossRef]
- Busch, A.; Herzer, T.; Waldegger, S.; Schmidt, F.; Palacin, M.; Biber, J.; Markovich, D.; Murer, H.; Lang, F. Opposite directed currents induced by the transport of dibasic and neutral amino acids in Xenopus oocytes expressing the protein rBAT. J. Biol. Chem. 1994, 269, 25581–25586. [Google Scholar] [PubMed]
- Reig, N.; Chillarón, J.; Bartoccioni, P.; Fernández, E.; Bendahan, A.; Zorzano, A.; Kanner, B.; Palacín, M.; Bertran, J. The light subunit of system bo,+ is fully functional in the absence of the heavy subunit. EMBO J. 2002, 21, 4906–4914. [Google Scholar] [CrossRef]
- Chillarón, J.; Estévez, R.; Mora, C.; A Wagner, C.; Suessbrich, H.; Lang, F.; Gelpí, J.L.; Testar, X.; E Busch, A.; Zorzano, A.; et al. Obligatory Amino Acid Exchange via Systems bo,+-like and y+L-like. J. Biol. Chem. 1996, 271, 17761–17770. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Cha, S.H.; Chairoungdua, A.; Kim, D.K.; Shigeta, Y.; Matsuo, H.; Fukushima, J.-I.; Awa, Y.; Akakura, K.; Goya, T.; et al. Human cystinuria-related transporter: Localization and functional characterization. Kidney Int. 2001, 59, 1821–1833. [Google Scholar] [CrossRef][Green Version]
- Bauch, C.; Verrey, F. Apical heterodimeric cystine and cationic amino acid transporter expressed in MDCK cells. Am. J. Physiol. Physiol. 2002, 283, F181–F189. [Google Scholar] [CrossRef]
- Crawhall, J.C.; Scowen, E.F.; Thompson, C.J.; Watts, R.W.E. The Renal Clearance of Amino Acids in Cystinuria. J. Clin. Investig. 1967, 46, 1162–1171. [Google Scholar] [CrossRef]
- Andreassen, K.H.; Pedersen, K.V.; Osther, S.S.; Jung, H.U.; Lildal, S.K.; Osther, P.J.S. How should patients with cystine stone disease be evaluated and treated in the twenty-first century? Urolithiasis 2015, 44, 65–76. [Google Scholar] [CrossRef]
- Martell, H.J.; Wong, K.A.; Martin, J.F.; Kassam, Z.; Thomas, K.; Wass, M.N. Associating mutations causing cystinuria with disease severity with the aim of providing precision medicine. BMC Genom. 2017, 18, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Xia, S.; Wu, Y.-S.; Di Paola, M.; Kissoon, R.; Luk, C.; Lin, F.; Du, K.; Rommens, J.M.; Bear, C.E. SLC6A14, an amino acid transporter, modifies the primary CF defect in fluid secretion. eLife 2018, 7, e37963. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, M.; Mercier, J.; Calmel, C.; Mésinèle, J.; Bigot, J.; Sutanto, E.N.; Kicic, A.; Corvol, H.; Guillot, L. Update on SLC6A14 in lung and gastrointestinal physiology and physiopathology: Focus on cystic fibrosis. Cell. Mol. Life Sci. 2020, 77, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.V.; Musante, L.; Romio, L.; Caruso, U.; Fantasia, A.; Gazzolo, A.; Romano, L.; Sacco, O.; Rossi, G.A.; Varesio, L.; et al. An electrogenic amino acid transporter in the apical membrane of cultured human bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L917–L923. [Google Scholar] [CrossRef] [PubMed]
- Jain-Vakkalagadda, B.; Pal, D.; Gunda, S.; Nashed, Y.; Ganapathy, V.; Mitra, A.K. Identification of a Na+-Dependent Cationic and Neutral Amino Acid Transporter, B0,+, in Human and Rabbit Cornea. Mol. Pharm. 2004, 1, 338–346. [Google Scholar] [CrossRef]
- Dai, R.; Peng, F.; Xiao, X.; Gong, X.; Jiang, Y.; Zhang, M.; Tian, Y.; Xu, Y.; Ma, J.; Li, M.; et al. Hepatitis B virus X protein-induced upregulation of CAT-1 stimulates proliferation and inhibits apoptosis in hepatocellular carcinoma cells. Oncotarget 2017, 8, 60962–60974. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Wang, J.; Yang, C.; Mao, H.; Fu, X.; Wu, Y.; Cai, J.; Han, J.; Xu, Z.; et al. Overexpression of Arginine Transporter CAT-1 Is Associated with Accumulation of L-Arginine and Cell Growth in Human Colorectal Cancer Tissue. PLoS ONE 2013, 8, e73866. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Rickard, J.A.; McDonald, W.J.; Thomas, L.N.; Too, C.K. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J. Cell. Biochem. 2011, 112, 1084–1092. [Google Scholar] [CrossRef]
- Deves, R.; Angelo, S.; Chávez, P. N-ethylmaleimide discriminates between two lysine transport systems in human erythrocytes. J. Physiol. 1993, 468, 753–766. [Google Scholar] [CrossRef]
- White, M.F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim. Biophys. Acta Rev. Biomembr. 1985, 822, 355–374. [Google Scholar] [CrossRef]
- White, M.F.; Gazzola, G.C.; Christensen, H.N. Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J. Biol. Chem. 1982, 257, 4443–4449. [Google Scholar] [PubMed]
- Wang, H.; Kavanaugh, M.P.; North, R.A.; Kabat, D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nat. Cell Biol. 1991, 352, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.R.; Mallmann, R.T.; Jaenecke, I.; Habermeier, A.; Boissel, J.-P.; Closs, E.I. Identification of Cysteine Residues in Human Cationic Amino Acid Transporter hCAT-2A That Are Targets for Inhibition byN-Ethylmaleimide. J. Biol. Chem. 2013, 288, 30411–30419. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.L.; Finley, K.D.; Kakuda, D.K. y(+)-type cationic amino acid transport: Expression and regulation of the mCAT genes. J. Exp. Biol. 1994, 196, 109–121. [Google Scholar]
- Shen, L.; Qian, C.; Cao, H.; Wang, Z.; Luo, T.; Liang, C. Upregulation of the solute carrier family 7 genes is indicative of poor prognosis in papillary thyroid carcinoma. World J. Surg. Oncol. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Hosokawa, H.; Sawamura, T.; Kobayashi, S.; Ninomiya, H.; Miwa, S.; Masaki, T. Cloning and Characterization of a Brain-specific Cationic Amino Acid Transporter. J. Biol. Chem. 1997, 272, 8717–8722. [Google Scholar] [CrossRef]
- Palacín, M.; Mora, C.; Chillarón, J.; Calonge, M.J.; Estévez, R.; Torrents, D.; Testar, X.; Zorzano, A.; Nunes, V.; Purroy, J.; et al. The molecular basis of cystinuria: The role of the rBAT gene. Amino Acids 1996, 11, 225–246. [Google Scholar]
- Jiang, Y.; Cao, Y.; Wang, Y.; Li, W.; Liu, X.; Lv, Y.; Li, X.; Mi, J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 2017, 7, 1036–1046. [Google Scholar] [CrossRef]
- Waldegger, S.; Schmidt, F.; Herzer, T.; Gulbins, E.; Schuster, A.; Biber, J.; Markovich, D.; Murer, H.; Busch, A.E.; Lang, F. Heavy metal mediated inhibition of rBAT-induced amino acid transport. Kidney Int. 1995, 47, 1677–1681. [Google Scholar] [CrossRef][Green Version]
- Pfeiffer, R.; Loffing, J.; Rossier, G.; Bauch, C.; Meier, C.; Eggermann, T.; Loffing-Cueni, M.; Kühn, L.C.; Verrey, F. Luminal Heterodimeric Amino Acid Transporter Defective in Cystinuria. Mol. Biol. Cell 1999, 10, 4135–4147. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Segawa, H.; Kim, J.Y.; Miyamoto, K.-I.; Haga, H.; Fukui, Y.; Mizoguchi, K.; Ito, H.; Takeda, E.; Endou, H.; et al. Identification of an Amino Acid Transporter Associated with the Cystinuria-related Type II Membrane Glycoprotein. J. Biol. Chem. 1999, 274, 28845–28848. [Google Scholar] [CrossRef] [PubMed]
- Bertran, J.; Werner, A.; Moore, M.L.; Stange, G.; Markovich, D.; Biber, J.; Testar, X.; Zorzano, A.; Palacín, M.; Murer, H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc. Natl. Acad. Sci. USA 1992, 89, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. y+LAT1 and y+LAT2 contribution to arginine uptake in different human cell models: Implications in the pathophysiology of Lysinuric Protein Intolerance. J. Cell. Mol. Med. 2020, 24, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Torrents, D.; Mykkänen, J.; Pineda, M.; Feliubadaló, L.; Estévez, R.; De Cid, R.; Sanjurjo, P.; Zorzano, A.; Nunes, V.; Huoponen, K.; et al. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat. Genet. 1999, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.P.; Annunziata, P.; Bozzato, A.; Piccolo, P.; Maiuri, L.; D’Armiento, M.; Ballabio, A.; Corso, G.; Andria, G.; Borsani, G.; et al. Slc7a7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am. J. Physiol. Physiol. 2007, 293, C191–C198. [Google Scholar] [CrossRef]
- Gunturiz, M.L.; Forrero, A.Y.; Chaparro, P.E. Genes Implicated in Obesity and Overweight: Potential Biomarkers of Early Diagnosis. Curr. Res. Clin. Diab. Obes. 2018. [Google Scholar] [CrossRef]
- Hu, X.; Han, T.; Bian, Y.; Tong, H.; Wen, X.; Li, Y.; Wan, X. Knockdown of SLCO4C1 inhibits cell proliferation and metastasis in endometrial cancer through inactivating the PI3K/Akt signaling pathway. Oncol. Rep. 2020, 43, 919–929. [Google Scholar] [CrossRef]
- Du, P.; Wang, A.; Ma, Y.; Jia, A.; Li, Y.; Li, X. Impact of SLCO4C1 Genotypes, Creatinine, and Spironolactone on Digoxin Population Pharmacokinetic Variables in Patients With Cardiac Insufficiency. Clin. Ther. 2020. [Google Scholar] [CrossRef]
- Sato, T.; Mishima, E.; Mano, N.; Abe, T.; Yamaguchi, H. Potential Drug Interactions Mediated by Renal Organic Anion Transporter OATP4C1. J. Pharmacol. Exp. Ther. 2017, 362, 271–277. [Google Scholar] [CrossRef]
- Taghikhani, E.; Maas, R.; Fromm, M.F.; König, J. The renal transport protein OATP4C1 mediates uptake of the uremic toxin asymmetric dimethylarginine (ADMA) and efflux of cardioprotective L-homoarginine. PLoS ONE 2019, 14, e0213747. [Google Scholar] [CrossRef]
- Taghikhani, E.; Maas, R.; Taudte, R.V.; Gessner, A.; Fromm, M.F.; König, J. Vectorial transport of the arginine derivatives asymmetric dimethylarginine (ADMA) and l-homoarginine by OATP4C1 and P-glycoprotein studied in double-transfected MDCK cells. Amino Acids 2020, 52, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.J.; Hu, T.; Wang, J. Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics. Pharmacol. Res. 2016, 111, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Schömig, E.; Lazar, A.; Gründemann, D. Extraneuronal Monoamine Transporter and Organic Cation Transporters 1 and 2: A Review of Transport Efficiency. Bone Regulators Osteop. Ther. 2006, 151–180. [Google Scholar] [CrossRef]
- Hacker, K.; Maas, R.; Kornhuber, J.; Fromm, M.F.; Zolk, O. Substrate-Dependent Inhibition of the Human Organic Cation Transporter OCT2: A Comparison of Metformin with Experimental Substrates. PLoS ONE 2015, 10, e0136451. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T.; Koepsell, H.; Damme, K.; Schwab, M. Organic Cation Transporters (OCTs, MATEs), In Vitro and In Vivo Evidence for the Importance in Drug Therapy. Bone Regulators Osteoporos. Ther. 2010, 105–167. [Google Scholar] [CrossRef]
- Cohen, R.; Basel-Vanagaite, L.; Goldberg-Stern, H.; Halevy, A.; Shuper, A.; Feingold-Zadok, M.; Behar, D.M.; Straussberg, R.; Basel-Salmon, L. Two siblings with early infantile myoclonic encephalopathy due to mutation in the gene encoding mitochondrial glutamate/H+ symporter SLC25A22. Eur. J. Paediatr. Neurol. 2014, 18, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Qian, Y.; Li, X.; Xu, J.; Kang, W.; Tong, J.H.; To, K.-F.; Jin, Y.; Li, W.; Chen, H.; et al. SLC25A22 Promotes Proliferation and Survival of Colorectal Cancer Cells With KRAS Mutations and Xenograft Tumor Progression in Mice via Intracellular Synthesis of Aspartate. Gastroenterology 2016, 151, 945–960. [Google Scholar] [CrossRef]
- Fiermonte, G.; Dolce, V.; David, L.; Santorelli, F.M.; Dionisi-Vici, C.; Palmieri, F.; Walker, J.E. The Mitochondrial Ornithine Transporter: Bacterial Expression, Reconstitution, Functional Characterization, and Tissue Distribution of Two Human Isoforms. J. Biol. Chem. 2003, 278, 32778–32783. [Google Scholar] [CrossRef]
- Porcelli, V.; Longo, A.; Palmieri, F.; Closs, E.I.; Palmieri, F. Asymmetric dimethylarginine is transported by the mitochondrial carrier SLC25A2. Amino Acids 2015, 48, 427–436. [Google Scholar] [CrossRef]
- Martinelli, D.; Diodato, N.; Ponzi, E.; Monné, M.; Boenzi, S.; Bertini, E.S.; Fiermonte, G.; Dionisi-Vici, C.C. The hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Orphanet J. Rare Dis. 2015, 10, 29. [Google Scholar] [CrossRef]
- Camacho, J.; Rioseco-Camacho, N. The Human and Mouse SLC25A29 Mitochondrial Transporters Rescue the Deficient Ornithine Metabolism in Fibroblasts of Patients with the Hyperornithinemia-Hyperammonemia-Homocitrullinuria (HHH) Syndrome. Pediatr. Res. 2009, 66, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. Diseases caused by defects of mitochondrial carriers: A review. Biochim. Biophys. Acta Gen. Subj. 2008, 1777, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, V.; Fiermonte, G.; Longo, A.; Palmieri, F. The Human GeneSLC25A29, of Solute Carrier Family 25, Encodes a Mitochondrial Transporter of Basic Amino Acids. J. Biol. Chem. 2014, 289, 13374–13384. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Nakamuta, S.; Miura, K.; Hirose, M.; Shiura, H.; Kohda, T.; Nakamuta, N.; Ogura, A. Paternal knockout of Slc38a4/SNAT4 causes placental hypoplasia associated with intrauterine growth restriction in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 21047–21053. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef]
- Christensen, H.N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 1990, 70, 43–77. [Google Scholar] [CrossRef]
- Hatanaka, T.; Huang, W.; Ling, R.; Prasad, P.D.; Sugawara, M.; Leibach, F.H.; Ganapathy, V. Evidence for the transport of neutral as well as cationic amino acids by ATA3, a novel and liver-specific subtype of amino acid transport system A. Biochim. Biophys. Acta Biomembr. 2001, 1510, 10–17. [Google Scholar] [CrossRef]
- Hägglund, M.G.A.; Sreedharan, S.; Nilsson, V.C.O.; Shaik, J.H.A.; Almkvist, I.M.; Bäcklin, S.; Wrange, O.; Fredriksson, R. Identification of SLC38A7 (SNAT7) Protein as a Glutamine Transporter Expressed in Neurons. J. Biol. Chem. 2011, 286, 20500–20511. [Google Scholar] [CrossRef]
- Poulter, J.A.; Al-Araimi, M.; Conte, I.; Van Genderen, M.M.; Sheridan, E.; Carr, I.M.; Parry, D.A.; Shires, M.; Carrella, S.; Bradbury, J.; et al. Recessive Mutations in SLC38A8 Cause Foveal Hypoplasia and Optic Nerve Misrouting without Albinism. Am. J. Hum. Genet. 2013, 93, 1143–1150. [Google Scholar] [CrossRef]
- Hägglund, M.G.; Hellsten, S.V.; Bagchi, S.; Philippot, G.; Löfqvist, E.; Nilsson, V.C.; Almkvist, I.; Karlsson, E.; Sreedharan, S.; Tafreshiha, A.; et al. Transport of l-Glutamine, l-Alanine, l-Arginine and l-Histidine by the Neuron-Specific Slc38a8 (SNAT8) in CNS. J. Mol. Biol. 2015, 427, 1495–1512. [Google Scholar] [CrossRef]
- Nies, A.T.; Damme, K.; Kruck, S.; Schaeffeler, E.; Schwab, M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch. Toxicol. 2016, 90, 1555–1584. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Inui, K.-I. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, H. Importance of P-glycoprotein for Drug–Drug Interactions. Bone Regulators Osteoporos. Ther. 2010, 201, 285–297. [Google Scholar] [CrossRef]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef]
- Kim, R.B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab. Rev. 2002, 34, 47–54. [Google Scholar] [CrossRef]
- Yasui-Furukori, N.; Uno, T. Drug-Drug Interactions of P-gp Substrates Unrelated to CYP Metabolism. Curr. Drug Metab. 2019, 20, 124–129. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef]
- Mikkaichi, T.; Suzuki, T.; Onogawa, T.; Tanemoto, M.; Mizutamari, H.; Okada, M.; Chaki, T.; Masuda, S.; Tokui, T.; Eto, N.; et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc. Natl. Acad. Sci. USA 2004, 101, 3569–3574. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Archiv Eur. J. Physiol. 2004, 447, 653–665. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Bleasby, K.; Yabut, J.; Cai, X.; Chan, G.H.; Hafey, M.J.; Xu, S.; Bergman, A.J.; Braun, M.P.; Dean, D.C.; et al. Transport of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin by Human Organic Anion Transporter 3, Organic Anion Transporting Polypeptide 4C1, and Multidrug Resistance P-glycoprotein. J. Pharmacol. Exp. Ther. 2007, 321, 673–683. [Google Scholar] [CrossRef]
- Suzuki, T.; Toyohara, T.; Akiyama, Y.; Takeuchi, Y.; Mishima, E.; Suzuki, C.; Ito, S.; Soga, T.; Abe, T. Transcriptional Regulation of Organic Anion Transporting Polypeptide SLCO4C1 as a New Therapeutic Modality to Prevent Chronic Kidney Disease. J. Pharm. Sci. 2011, 100, 3696–3707. [Google Scholar] [CrossRef] [PubMed]
- AlbarracÌn, M.G.; Forero, A.Y.; Chaparro, P. Genes implicated in obesity and overweight potential biomarkers of early diagnosis. Curr. Res. Clin. Diab. Obes. 2018, 2018. [Google Scholar] [CrossRef]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Volk, C. OCTs, OATs, and OCTNs: Structure and function of the polyspecific organic ion transporters of the SLC22 family. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Taubert, D.; Grimberg, G.; Stenzel, W.; Schömig, E. Identification of the Endogenous Key Substrates of the Human Organic Cation Transporter OCT2 and Their Implication in Function of Dopaminergic Neurons. PLoS ONE 2007, 2, e385. [Google Scholar] [CrossRef]
- Gorboulev, V.; Ulzheimer, J.C.; Akhoundova, A.; Ulzheimer-Teuber, I.; Karbach, U.; Quester, S.; Baumann, C.; Lang, F.; Busch, A.E.; Koepsell, H. Cloning and Characterization of Two Human Polyspecific Organic Cation Transporters. DNA Cell Biol. 1997, 16, 871–881. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Schinkel, A.H.; et al. Organic Cation Transporter 2 Mediates Cisplatin-Induced Oto- and Nephrotoxicity and Is a Target for Protective Interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human Neurons Express the Polyspecific Cation Transporter hOCT2, Which Translocates Monoamine Neurotransmitters, Amantadine, and Memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef]
- Harper, J.N.; Wright, S.H. Multiple mechanisms of ligand interaction with the human organic cation transporter, OCT2. Am. J. Physiol. Physiol. 2013, 304, F56–F67. [Google Scholar] [CrossRef]
- Gründemann, D.; Köster, S.; Kiefer, N.; Breidert, T.; Engelhardt, M.; Spitzenberger, F.; Obermüller, N.; Schömig, E. Transport of Monoamine Transmitters by the Organic Cation Transporter Type 2, OCT2. J. Biol. Chem. 1998, 273, 30915–30920. [Google Scholar] [CrossRef]
- Leone, A.; Moncada, S.; Vallance, P.; Calver, A.; Collier, J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Lai, Y.; Varma, M.; Feng, B.; Stephens, J.C.; Kimoto, E.; El-Kattan, A.; Ichikawa, K.; Kikkawa, H.; Ono, C.; Suzuki, A.; et al. Impact of drug transporter pharmacogenomics on pharmacokinetic and pharmacodynamic variability—Considerations for drug development. Expert Opin. Drug Metab. Toxicol. 2012, 8, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; Kunji, E.R.S. The SLC25 Mitochondrial Carrier Family: Structure and Mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.A.; Obie, C.; Biery, B.; Goodman, B.K.; Hu, C.-A.; Almashanu, S.; Steel, G.; Casey, R.; Lambert, M.; Mitchell, G.A.; et al. Hyperornithinaemia- hyperammonaemia- homocitrullinuria syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat. Genet. 1999, 22, 151–158. [Google Scholar] [CrossRef]
- A Camacho, J.; Rioseco-Camacho, N.; Andrade, D.; Porter, J.; Kong, J. Cloning and characterization of human ORNT2: A second mitochondrial ornithine transporter that can rescue a defective ORNT1 in patients with the hyperornithinemia–hyperammonemia–homocitrullinuria syndrome, a urea cycle disorder. Mol. Genet. Metab. 2003, 79, 257–271. [Google Scholar] [CrossRef]
- Sekoguchi, E.; Sato, N.; Yasui, A.; Fukada, S.; Nimura, Y.; Aburatani, H.; Ikeda, K.; Matsuura, A. A Novel Mitochondrial Carnitine-acylcarnitine Translocase Induced by Partial Hepatectomy and Fasting. J. Biol. Chem. 2003, 278, 38796–38802. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Palmieri, L.; De Marco, V.; Iacobazzi, V.; Palmieri, F.; Runswick, M.J.; Walker, J.E. Identification of the yeast ARG-11 gene as a mitochondrial ornithine carrier involved in arginine biosynthesis. FEBS Lett. 1997, 410, 447–451. [Google Scholar] [CrossRef]
- MacKenzie, B.; Erickson, J.D. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflügers Archiv Eur. J. Physiol. 2004, 447, 784–795. [Google Scholar] [CrossRef]
- Desforges, M.; Lacey, H.A.; Glazier, J.D.; Greenwood, S.L.; Mynett, K.J.; Speake, P.F.; Sibley, C.P. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am. J. Physiol. Physiol. 2006, 290, C305–C312. [Google Scholar] [CrossRef]
- Jackson, M.J.; Beaudet, A.L.; E O’Brien, W. Mammalian Urea Cycle Enzymes. Annu. Rev. Genet. 1986, 20, 431–464. [Google Scholar] [CrossRef] [PubMed]
- Damme, K.; Nies, A.T.; Schaeffeler, E.; Schwab, M. Mammalian MATE (SLC47A) transport proteins: Impact on efflux of endogenous substrates and xenobiotics. Drug Metab. Rev. 2011, 43, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Yasuda, M.; Morita, Y.; Otsuka, C.; Tsuchiya, T.; Omote, H.; Moriyama, Y. Identification of Essential Amino Acid Residues of the NorM Na+/Multidrug Antiporter in Vibrio parahaemolyticus. J. Bacteriol. 2005, 187, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K.-I. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Terada, T.; Asaka, J.-I.; Ueba, M.; Katsura, T.; Inui, K.-I. Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. Am. J. Physiol. Physiol. 2007, 292, F593–F598. [Google Scholar] [CrossRef]
- König, J.; Zolk, O.; Singer, K.; Hoffmann, C.; Fromm, M.F. Double-transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: Determinants of uptake and transcellular translocation of organic cations. Br. J. Pharmacol. 2011, 163, 546–555. [Google Scholar] [CrossRef]
- Vallance, P.; Leiper, J. Cardiovascular Biology of the Asymmetric Dimethylarginine:Dimethylarginine Dimethylaminohydrolase Pathway. Arter. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef]
- Juliano, R.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef]
- Meissner, K.; Sperker, B.; Karsten, C.; Zu Schwabedissen, H.E.M.; Seeland, U.; Böhm, M.; Bien, S.; Dazert, P.; Kunert-Keil, C.; Vogelgesang, S.; et al. Expression and Localization of P-glycoprotein in Human Heart. J. Histochem. Cytochem. 2002, 50, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Hunter, J.; Hirst, B.H. Intestinal secretion of drugs. The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv. Drug Deliv. Rev. 1997, 25, 129–157. [Google Scholar] [CrossRef]
- Fromm, M.F. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and Function of P-Glycoprotein in Normal Tissues: Effect on Pharmacokinetics. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin, Germany, 2009; Volume 596, pp. 199–222. [Google Scholar]
- Parker, R.B.; Yates, C.R.; Laizure, S.C.; Weber, K.T. P-Glycoprotein Modulates Aldosterone Plasma Disposition and Tissue Uptake. J. Cardiovasc. Pharmacol. 2006, 47, 55–59. [Google Scholar] [CrossRef]
- Eap, C.B.; Bochud, M.; Elston, R.C.; Bovet, P.; Maillard, M.P.; Nussberger, J.; Schild, L.; Shamlaye, C.; Burnier, M. CYP3A5 and ABCB1 Genes Influence Blood Pressure and Response to Treatment, and Their Effect Is Modified by Salt. Hypertension 2007, 49, 1007–1014. [Google Scholar] [CrossRef]
- Bochud, M.; Eap, C.B.; Maillard, M.P.; Johnson, T.; Vollenweider, P.; Bovet, P.; Elston, R.C.; Bergmann, S.; Beckmann, J.S.; Waterworth, D.; et al. Association of ABCB1 genetic variants with renal function in Africans and in Caucasians. BMC Med. Genom. 2008, 1, 21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).