Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Data Collection
3. Laboratory Analysis
3.1. Assay Validation
3.2. Statistical Methods
4. Results
4.1. Baseline Characteristics
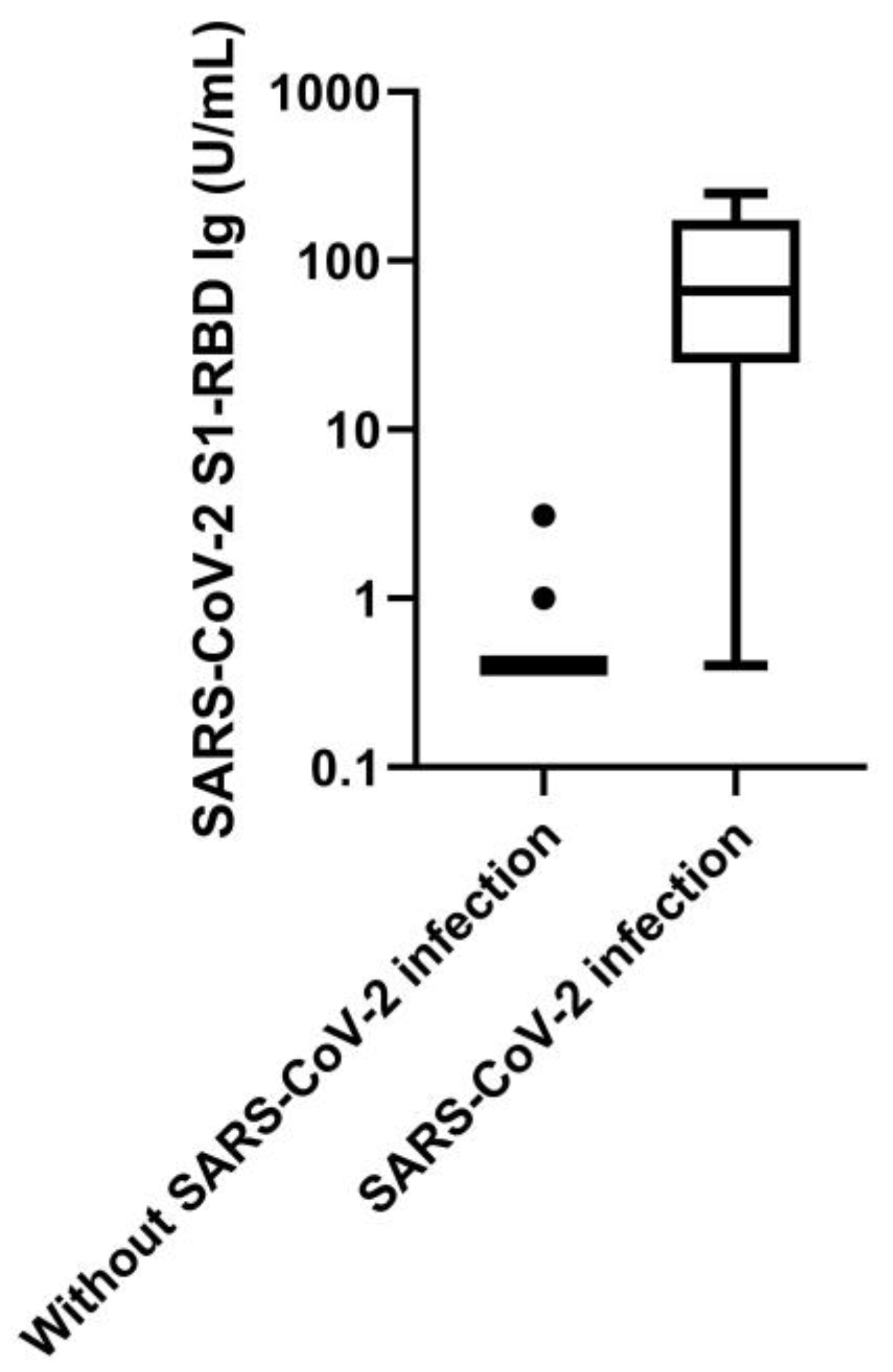
4.2. Assay Validation
4.3. Analytic Specificity
4.4. Diagnostic Specificity and Sensitivity at the Manufacturers’ Cutoff
4.5. Association of Pan-SARS-CoV-2 S1-RBD with Clinical Variables
4.6. Kinetics of SARS-CoV-2 S1-RBD Antibodies
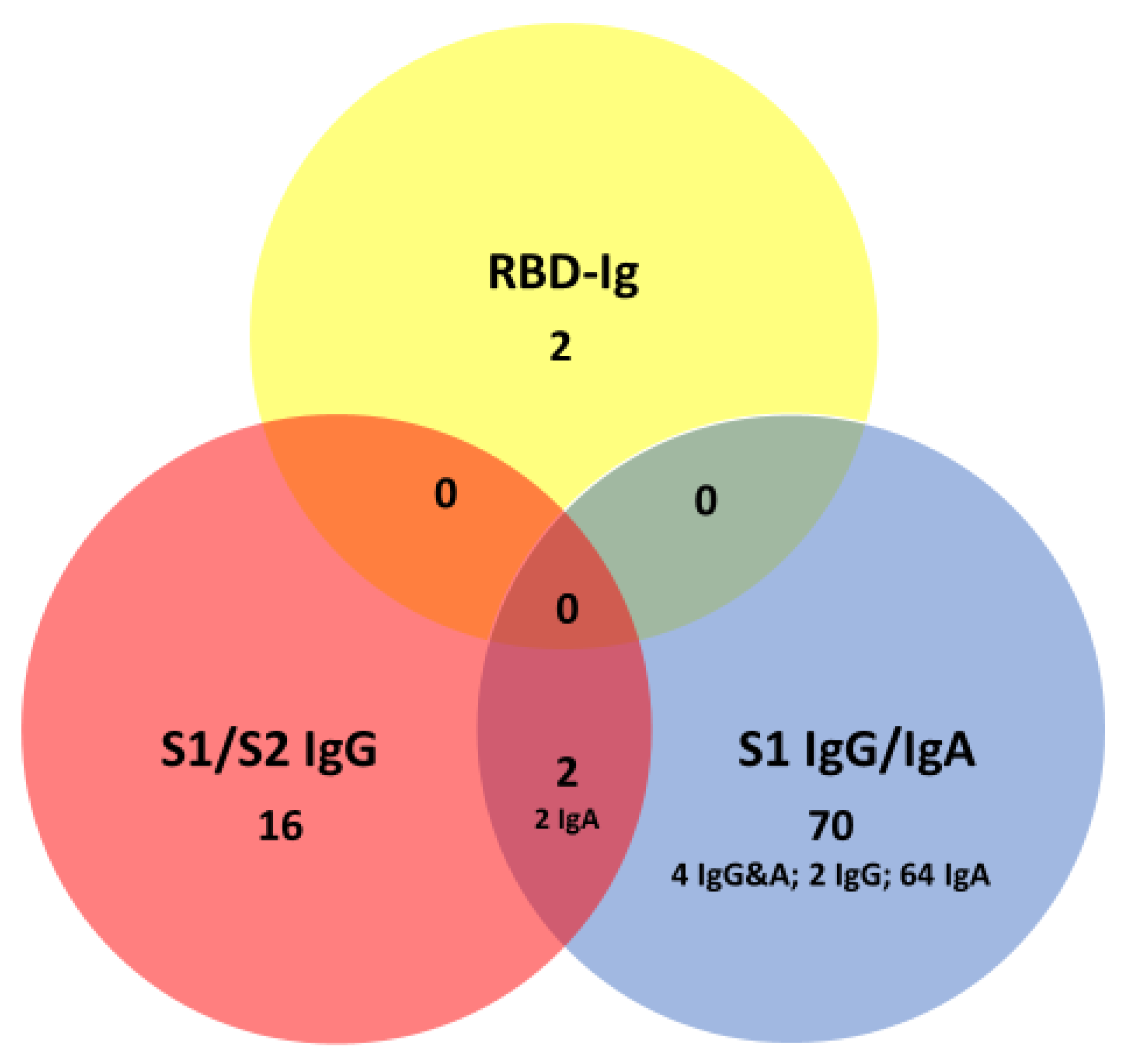
4.7. Sensitivity of Other Anti-Spike Protein Antibodies in Patients with SARS_CoV-2 Infection
4.8. Specificity of Anti-Spike Protein Antibodies in Patients without SARS-CoV-2 Infection
4.9. Positive and Negative Predictive Values
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Patel, R.; Babady, N.E.; Theel, E.S.; Storch, G.A.; Pinsky, B.A.; George, K.S.; Smith, T.C.; Bertuzzi, S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS–CoV-2/COVID-19. mBio 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Baron, R.C.; Risch, L.; Weber, M.; Thiel, S.; Grossmann, K.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Bigler, S.; et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: A population-based study. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bhagvathula, A.S.; AlDhaleei, W.A.; Rahmani, J.; Mahabadi, M.A.; Bandari, D.K. Knowledge and Perceptions of COVID-19 Among Health Care Workers: Cross-Sectional Study. JMIR Public Health Surveill. 2020, 6, e19160. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.L.; Weber, M.C.; Risch, L.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Risch, M.; Kohler, P.; Vernazza, P.; et al. Flattening the curve in 52 days: Characterisation of the COVID-19 pandemic in the Principality of Liechtenstein—An observational study. Swiss Med. Wkly. 2020, 150, w2036. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.K.; Hegde, S.T. The important role of serology for COVID-19 control. Lancet Infect. Dis. 2020, 20, 758–759. [Google Scholar] [CrossRef]
- Pung, R.; Chiew, C.J.; Young, B.E.; Chin, S.; Chen, M.I.-C.; Clapham, H.E.; Cook, A.R.; Maurer-Stroh, S.; Toh, M.P.H.S.; Poh, C.; et al. Investigation of three clusters of COVID-19 in Singapore: Implications for surveillance and response measures. Lancet 2020, 395, 1039–1046. [Google Scholar] [CrossRef]
- Yong, S.E.F.; Anderson, D.E.; Wei, W.E.; Pang, J.; Ni Chia, W.; Tan, C.W.; Teoh, Y.L.; Rajendram, P.; Toh, M.P.H.S.; Poh, C.; et al. Connecting clusters of COVID-19: An epidemiological and serological investigation. Lancet Infect. Dis. 2020, 20, 809–815. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. Interim Guidelines for COVID-19 Antibody Testing. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 30 October 2020).
- Lung, T.; Kazatchkine, M.D.; Risch, L.; Risch, M.; Nydegger, U. A consideration of convalescent plasma and plasma derivatives in the care of Severely-ill patients with COVID-19. Transfus. Apher. Sci. 2020, 2020, 102936. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.A.; Senefeld, J.W.; Johnson, P.W.; Carter, R.E.; Wiggins, C.C.; Shoham, S.; Grossman, B.J.; Henderson, J.P.; Musser, J.; Salazar, E.; et al. Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv 2020. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: What’s going wrong with testing in the UK? BMJ 2020, 370, m3678. [Google Scholar] [CrossRef]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, A.; Risch, L.; Weber, M.; Thiel, S.; Juengert, K.; Pichler, M.; Wohlwend, N.; Lung, T.; Ritzler, M.; Hillmann, D.; et al. Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: A population-based study. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Shi, J.; Han, D.; Zhang, R.; Li, J.; Zhang, R. Molecular and Serological Assays for SARS-CoV-2: Insights from Genome and Clinical Characteristics. Clin. Chem. 2020, 66, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Z.; Ye, K.; He, X.; Sun, B.; Qin, Z.; Yu, J.; Yao, J.; Wu, Q.; Bao, Z.; et al. SARS-CoV-2: Characteristics and current advances in research. Virol. J. 2020, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pillay, T.S. Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020, 73, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nat. Cell Biol. 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nat. Cell Biol. 2020, 584, 115–119. [Google Scholar] [CrossRef]
- San Tang, M.; Case, J.B.; Franks, C.E.; Chen, R.E.; Anderson, N.W.; Henderson, J.P.; Farnsworth, C.W. Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays. Clin. Chem. 2020. [Google Scholar] [CrossRef]
- Rychert, J.; Couturier, M.R.; Elgort, M.; Lozier, B.K.; La’ulu, S.; Genzen, J.R.; Slev, P.R. Evaluation of Three SARS CoV-2 IgG Antibody Assays and Correlation with Neutralizing Antibodies. J. Appl. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Kovac, M.; Risch, L.; Thiel, S.; Weber, M.; Grossmann, K.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Bigler, S.; et al. EDTA-Anticoagulated Whole Blood for SARS-CoV-2 Antibody Testing by Electrochemiluminescence Immunoassay (ECLIA) and Enzyme-Linked Immunosorbent Assay (ELISA). Diagnostics 2020, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Defining the Role of a Fertility Bracelet for Early Recognition and Monitoring of COVID-19 in Liechtenstein: An Observational Study (COVI-GAPP) ISRCTN. 2020. Available online: http://www.isrctn.com/ISRCTN51255782 (accessed on 30 October 2020).
- Weber, M.C.; Risch, M.; Thiel, S.L.; Grossmann, K.; Nigg, S.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Ferrara, F.; et al. Characteristics of three different chemiluminescence assays for testing for SARS-CoV-2 antibodies. medRxiv 2020. [Google Scholar] [CrossRef]
- Pfefferle, S.; Reucher, S.; Nörz, D.; Lütgehetmann, M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eurosurveillance 2020, 25, 2000152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, P.; Kahlert, C.R.; Sumer, J.; Flury, D.; Güsewell, S.; Leal-Neto, O.B.; Notter, J.; Albrich, W.C.; Flury, B.B.; McGeer, A.; et al. Prevalence of SARS-CoV-2 Antibodies among Swiss Hospital Workers—Results of a Prospective Cohort Study. Infect. Control Hosp. Epidemiol. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Risch, L.; Monn, A.; Luthy, R.; Honegger, H.; Huber, A.R. The predictive characteristics of D-dimer testing in outpatients with suspected venous thromboembolism: A Bayesian approach. Clin. Chim. Acta 2004, 345, 79–87. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. EUA Authorized Serology Test Performance. 2020. Available online: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance (accessed on 11 July 2020).
- Amt für Statistik des Fuerstentum Liechtensteni. Bevoelkerungsstatistik per 31.12.2019; Amt für Statistik des Fuerstentum Liechtenstein: Vaduz, Liechtenstein, 2020. [Google Scholar]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Dogné, J.-M.; Douxfils, J.; Blairon, L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin. Chem. Lab. Med. 2020, 58, 1357–1364. [Google Scholar] [CrossRef]
- Plebani, M.; Padoan, A.; Negrini, D.; Carpinteri, B.; Sciacovelli, L. Diagnostic performances and thresholds: The key to harmonization in serological SARS-CoV-2 assays? Clin. Chim. Acta 2020, 509, 1–7. [Google Scholar] [CrossRef]
- Hanson, K.E.; Caliendo, A.M.; Arias, A.C.; Englund, A.J.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Kalot, A.M.; Falck-Ytter, Y.; et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Patel, M.M.; Thornburg, N.J.; Stubblefield, W.B.; Talbot, H.K.; Coughlin, M.M.; Feldstein, L.R.; Self, W.H. Change in Antibodies to SARS-CoV-2 Over 60 Days Among Health Care Personnel in Nashville, Tennessee. JAMA 2020. [Google Scholar] [CrossRef]
- Beaudoin-Bussières, G.; Laumaea, A.; Anand, S.P.; Prevost, J.; Gasser, R.; Goyette, G.; Medjahed, H.; Perreault, J.; Tremblay, T.; Lewin, A.; et al. Decline of humoral responses against SARS-CoV-2 Spike in convalescent individuals. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Risch, M.; Weber, M.; Thiel, S.; Grossmann, K.; Wohlwend, N.; Risch, T.; Hillmann, D.; Ritzler, M.; Ferrara, F.; Bigler, S.; et al. Temporal course of SARS-CoV-2 antibody positivity in patients with COVID-19 following the first clinical presentation. BioMed Res. Int. 2020, 2020, 9878453. [Google Scholar] [CrossRef] [PubMed]
- Korte, W.; Buljan, M.; Rösslein, M.; Wick, P.; Golubov, V.; Jentsch, J.; Reut, M.; Peier, K.; Nohynek, B.; Fischer, A.; et al. SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C. Evolution of Antibody Immunity to SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Simen-Kapeu, A.; Kataja, V.; Yliskoski, M.; Syrjänen, K.; Dillner, J.; Koskela, P.; Paavonen, J.; Lehtinen, M. Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scand. J. Infect. Dis. 2008, 40, 745–751. [Google Scholar] [CrossRef]
- Tarbiah, N.; Todd, I.; Tighe, P.J.; Fairclough, L.C. Cigarette smoking differentially affects immunoglobulin class levels in serum and saliva: An investigation and review. Basic Clin. Pharmacol. Toxicol. 2019, 125, 474–483. [Google Scholar] [CrossRef]
- Simons, D.; Shahab, L.; Brown, J.; Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: A living rapid evidence review with Bayesian meta-analyses (version 7). Qeios 2020. [Google Scholar] [CrossRef]
- Mesas, A.E.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Sarriá Cabrera, M.A.; Maffei de Andrade, S.; Sequí-Dominguez, I.; Martínez-Vizcaíno, V. Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS ONE 2020, 15, e0241742. [Google Scholar] [CrossRef]
- Criscuolo, E.; Diotti, R.A.; Strollo, M.; Rolla, S.; Ambrosi, A.; Locatelli, M.; Burioni, R.; Mancini, N.; Clementi, M.; Clementi, N. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Ng, K.; Faulkner, N.; Cornish, G.; Rosa, A.; Earl, C.; Wrobel, A.; Agua-Doce, A. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Tian, Y.; Lian, C.; Chen, Y.; Wei, D.; Zhang, X.; Ling, Y.; Wang, Y.; Yeap, L.S. Sensitivity and specificity of SARS-CoV-2 S1 subunit in COVID-19 serology assays. Cell Discov. 2020, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, G.; Schepp, R.M.; Kuijer, M.; GeurtsvanKessel, C.; Van Beek, J.; Rots, N.; Koopmans, M.P.G.; Van der Klis, F.R.M.; Van Binnendijk, R.S. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef]







| Cohort | Patients/Participants | Criteria for Infection | Criteria for Absence of Infection | Evaluation of |
|---|---|---|---|---|
| COVID-FL | COVID-19 index cases & Infected close contacts (n = 125) | History and RT-PCR positive History and serology positive | N/A | Diagnostic sensitivity |
| COVID-FL | Non-infected close contacts (n = 123) | N/A | RT-PCR and/or serology negative | Diagnostic specificity |
| COVI-GAPP | Non-infected participants (n = 1036) | N/A | Absent history and serology negative | Diagnostic specificity |
| Biobank samples | Pre-pandemic patients with EBV (n = 8), CMV (n = 7), HCoV (n = 12) | Laboratory confirmed infection | N/A | Analytic specificity |
| Symptom | Antibody Level in Patients with Symptom, U/mL Median (IQR) | Antibody Level in Patients without Symptom, U/mL Median (IQR) | p-Value |
|---|---|---|---|
| Cough | 79 (34–184) | 54 (10–149) | 0.17 |
| (n) | (80) | (45) | |
| Fever | 99 (53–205) | 46 (17–120) | 0.001 |
| (n) | (61) | (64) | |
| Dysgeusia | 62 (21–142) | 71 (33–178) | 0.58 |
| (n) | (59) | (66) | |
| Headache | 60 (20–189) | 66 (35–138) | 0.99 |
| (n) | (60) | (65) | |
| Fatigue | 73 (21–196) | 63 (33–149) | 0.75 |
| (n) | (59) | (66) | |
| Anosmia | 59 (25–118) | 73 (24–193) | 0.3 |
| (n) | (48) | (77) | |
| Bone, joint and muscle pain | 53 (17–119) | 76 (27–182) | 0.17 |
| (n) | (41) | (84) | |
| Rhinitis | 66 (22–128) | 62 (25–182) | 0.56 |
| (n) | (40) | (85) | |
| Sore throat | 79 (20–183) | 79 (34–184) | 0.81 |
| (n) | (38) | (87) | |
| Chest pain | 74 (25–250) | 61 (23–166) | 0.69 |
| (n) | (34) | (91) | |
| Dyspnea | 82 (22–250) | 62 (27–144) | 0.4 |
| (n) | (28) | (97) | |
| Diarrhea | 35 (13–128) | 38 (74–175) | 0.06 |
| (n) | (27) | (98) | |
| Malaise | 57 (14–116) | 76 (27–180) | 0.17 |
| (n) | (25) | (100) | |
| Nausea | 75 (41–99) | 63 (24–180) | 0.6 |
| (n) | (13) | (112) | |
| Smoking | 24 (7–80) | 70 (30–178) | 0.05 |
| (n) | (11) | (114) |
| Pretest Probability | S1-RBD-Ig PPV | S1-RBD-Ig NPV | S1-RBD-Ig and N-Ig PPV | S1-RBD-Ig and N-Ig NPV |
|---|---|---|---|---|
| 40% | 99.7% | 98.4% | 100% | 7.1% |
| 30% | 99.5% | 99% | 100% | 10.7% |
| 25% | 99.4% | 99.2% | 100% | 13.3% |
| 20% | 99.2% | 99.4% | 100% | 17% |
| 15% | 98.9% | 99.6% | 100% | 22.5% |
| 10% | 98.2% | 99.7% | 100% | 31.5% |
| 8% | 97.7% | 99.8% | 100% | 37% |
| 5% | 96.3% | 99.9% | 100% | 49.3% |
| 3% | 93.8% | 99.9% | 100% | 62.3% |
| 2% | 90.9% | 100% | 100% | 71.5% |
| 1% | 83.1% | 100% | 100% | 83.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaffner, A.; Risch, L.; Aeschbacher, S.; Risch, C.; Weber, M.C.; Thiel, S.L.; Jüngert, K.; Pichler, M.; Grossmann, K.; Wohlwend, N.; et al. Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. J. Clin. Med. 2020, 9, 3989. https://doi.org/10.3390/jcm9123989
Schaffner A, Risch L, Aeschbacher S, Risch C, Weber MC, Thiel SL, Jüngert K, Pichler M, Grossmann K, Wohlwend N, et al. Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. Journal of Clinical Medicine. 2020; 9(12):3989. https://doi.org/10.3390/jcm9123989
Chicago/Turabian StyleSchaffner, Anna, Lorenz Risch, Stefanie Aeschbacher, Corina Risch, Myriam C. Weber, Sarah L. Thiel, Katharina Jüngert, Michael Pichler, Kirsten Grossmann, Nadia Wohlwend, and et al. 2020. "Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study" Journal of Clinical Medicine 9, no. 12: 3989. https://doi.org/10.3390/jcm9123989
APA StyleSchaffner, A., Risch, L., Aeschbacher, S., Risch, C., Weber, M. C., Thiel, S. L., Jüngert, K., Pichler, M., Grossmann, K., Wohlwend, N., Lung, T., Hillmann, D., Bigler, S., Bodmer, T., Imperiali, M., Renz, H., Kohler, P., Vernazza, P., Kahlert, C. R., ... Risch, M. (2020). Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study. Journal of Clinical Medicine, 9(12), 3989. https://doi.org/10.3390/jcm9123989







