Kinetic GFR Outperforms CKD-EPI for Slow Graft Function Prediction in the Immediate Postoperative Period Following Kidney Transplantation
Abstract
:1. Introduction
2. Materials and Methods
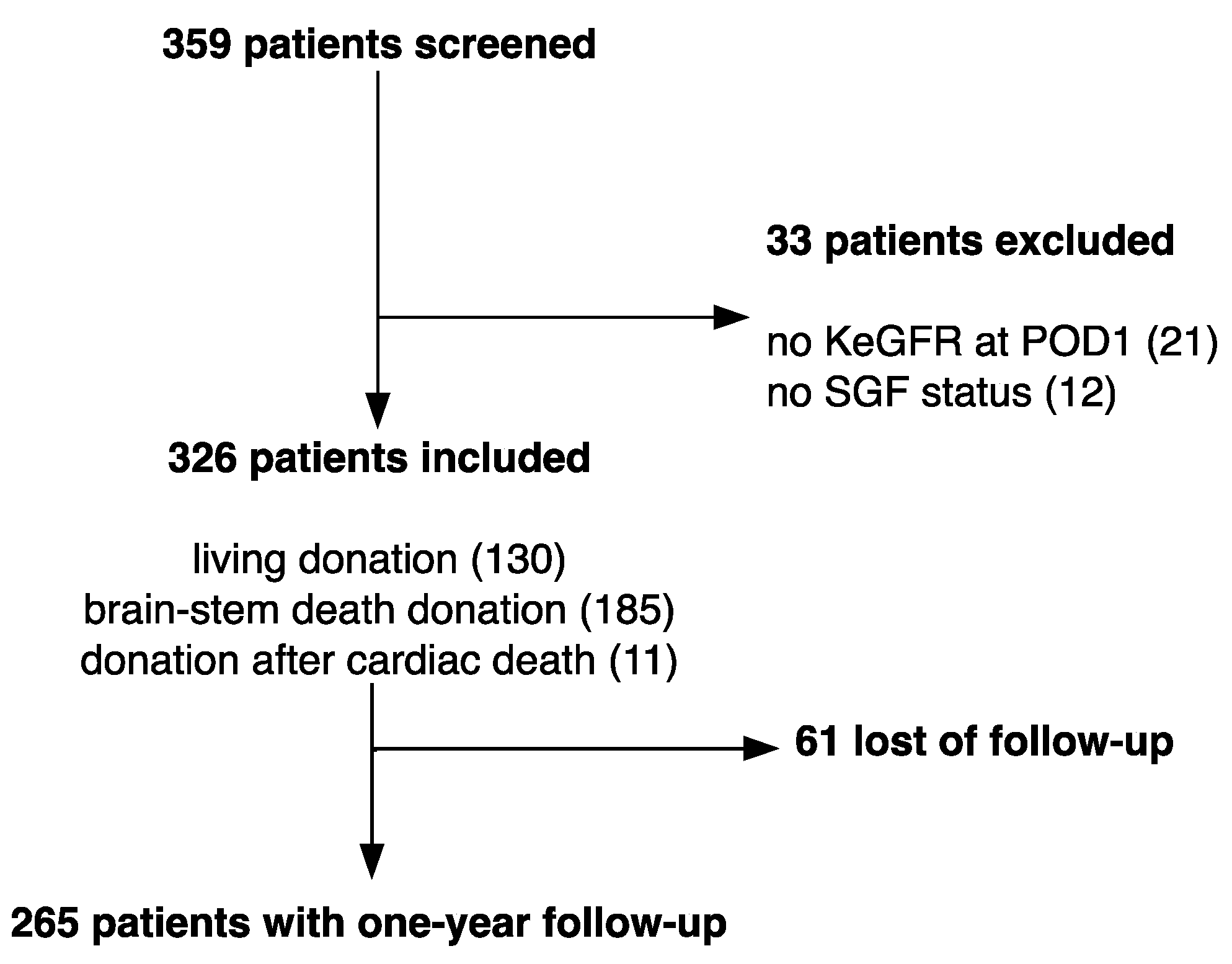
2.1. Study Design
2.2. Definitions
2.3. Data Sources
2.4. eGFR Calculations
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Outcomes
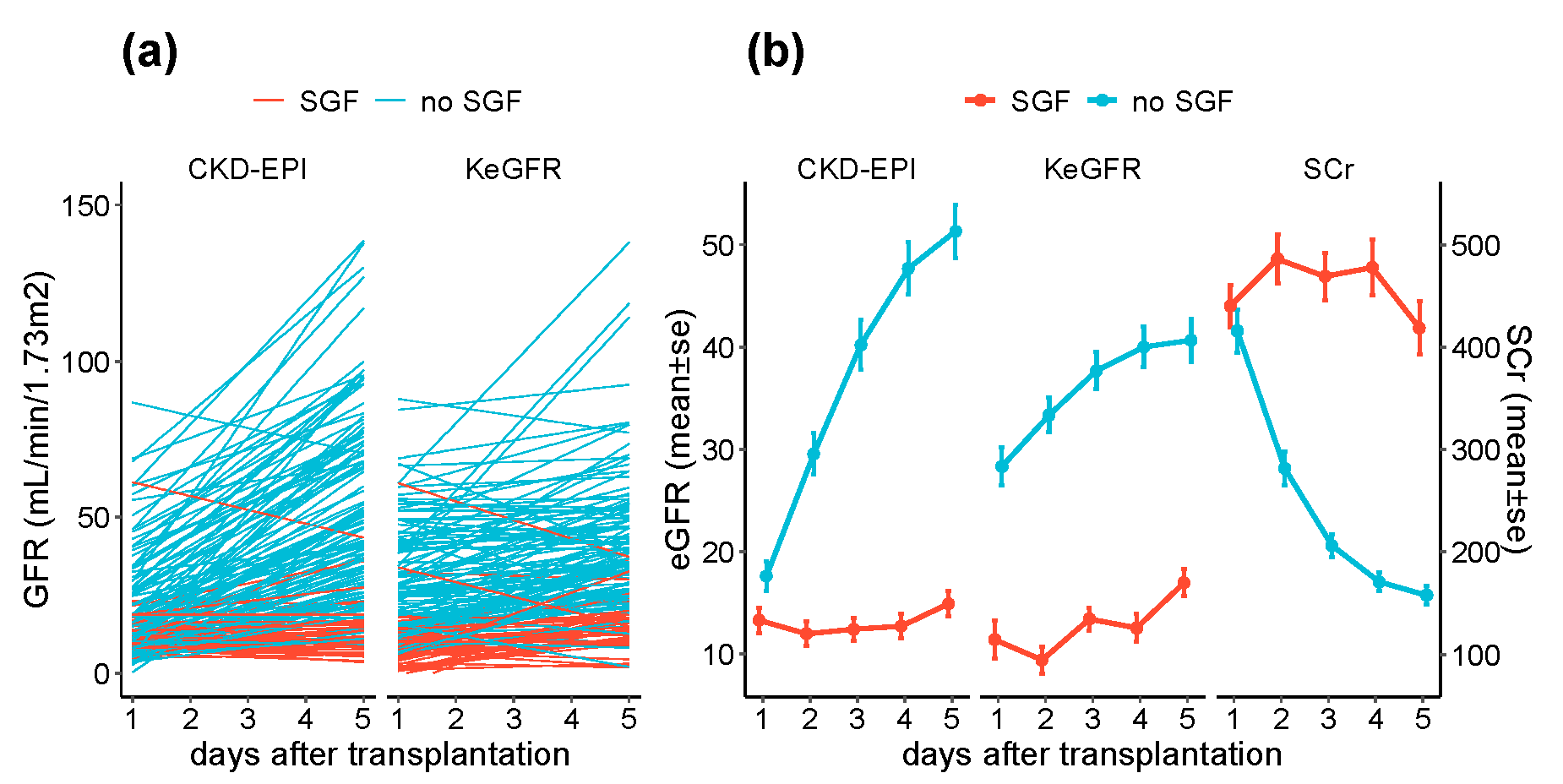
3.2. KeGFR and CKD-EPI in the Immediate Post-Transplantation Period
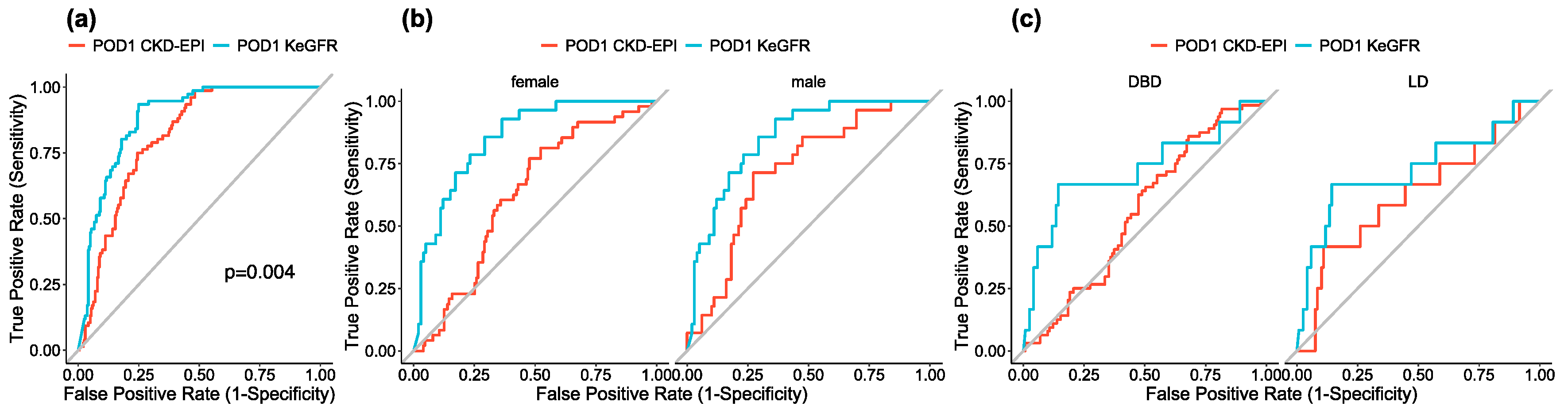
3.3. POD1 KeGFR and CKD-EPI According to SGF Status
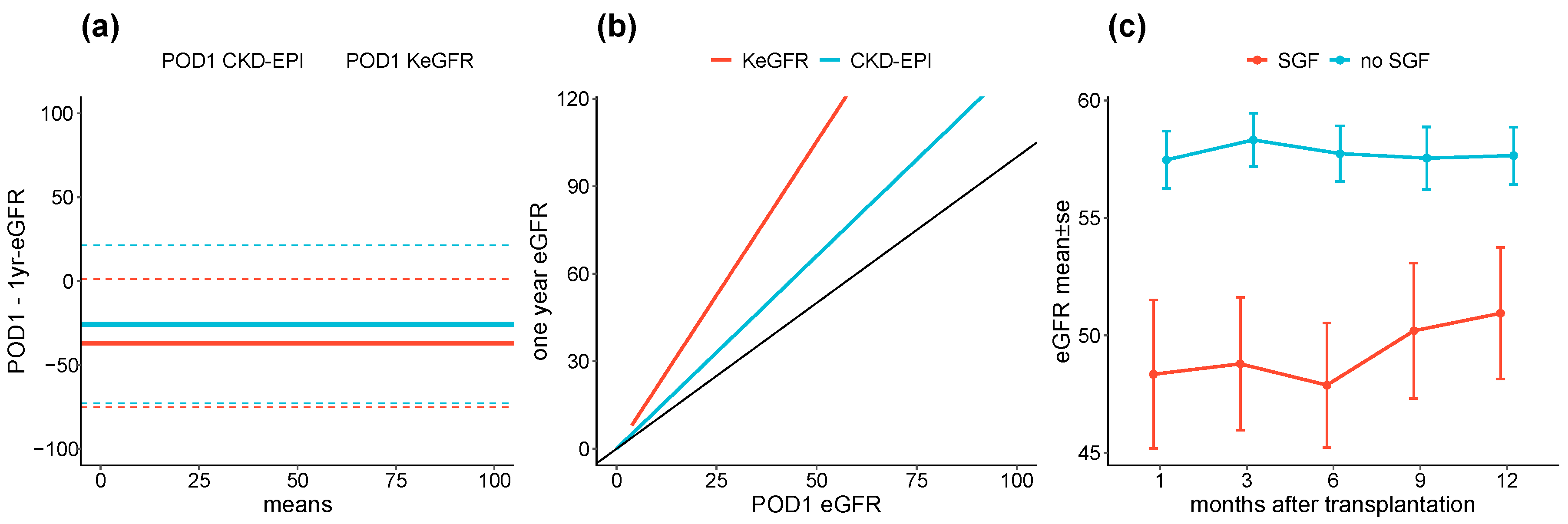
3.4. POD1 eGFR, SGF and Renal Function over Follow Up
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Yarlagadda, S.G.; Coca, S.G.; Garg, A.X.; Doshi, M.; Poggio, E.; Marcus, R.J.; Parikh, C.R. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol. Dial. Transplant. 2008, 23, 2995–3003. [Google Scholar] [CrossRef] [Green Version]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2009, 24, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Tapiawala, S.N.; Tinckam, K.J.; Cardella, C.J.; Schiff, J.; Cattran, D.C.; Cole, E.H.; Kim, S.J. Delayed graft function and the risk for death with a functioning graft. J. Am. Soc. Nephrol. 2010, 21, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Farney, A.C.; Rogers, J.; Zuckerman, J.; Reeves-Daniel, A.; Hartmann, E.; Iskandar, S.; Adams, P.; Stratta, R.J. Kidney transplantation from donation after cardiac death donors: Lack of impact of delayed graft function on post-transplant outcomes: No impact of DGF in DCD kidneys. Clin. Transplant. 2011, 25, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Garonzik Wang, J.M.; Massie, A.B.; Jackson, K.R.; McAdams-DeMarco, M.A.; Brennan, D.C.; Lentine, K.L.; Coresh, J.; Segev, D.L. Early steroid withdrawal in deceased-donor kidney transplant recipients with delayed graft function. J. Am. Soc. Nephrol. 2020, 31, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.J.; Smith, J.M.; Skeans, M.A.; Thompson, B.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Boyle, G.; Snyder, J.J.; et al. OPTN/SRTR 2013 Annual Data Report: Kidney: OPTN/SRTR 2013 Annual Data Report. Am. J. Transplant. 2015, 15, 1–34. [Google Scholar] [CrossRef]
- Redfield, R.R.; Scalea, J.R.; Zens, T.J.; Muth, B.; Kaufman, D.B.; Djamali, A.; Astor, B.C.; Mohamed, M. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl. Int. 2016, 29, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Johnson, E.M.; Payne, W.D.; Wrenshall, L.; Sutherland, D.E.; Najarian, J.S.; Gillingham, K.J.; Matas, A.J. Effect of initial slow graft function on renal allograft rejection and survival. Clin. Transplant. 1997, 11, 623–627. [Google Scholar] [PubMed]
- Humar, A.; Ramcharan, T.; Kandaswamy, R.; Gillingham, K.; Payne, W.D.; Matas, A.J. Risk factors for slow graft function after kidney transplants: A multivariate analysis. Clin. Transplant. 2002, 16, 425–429. [Google Scholar] [CrossRef]
- Wang, C.J.; Tuffaha, A.; Phadnis, M.A.; Mahnken, J.D.; Wetmore, J.B. Association of slow graft function with long-term outcomes in kidney transplant recipients. Ann. Transplant. 2018, 23, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.; Vanholder, R. Therapeutic interventions favorably influencing delayed and slow graft function in kidney transplantation: Mission impossible? Transplantation 2008, 85, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Pianta, T.J.; Peake, P.W.; Pickering, J.W.; Kelleher, M.; Buckley, N.A.; Endre, Z.H. Clusterin in Kidney Transplantation: Novel biomarkers versus serum creatinine for early prediction of delayed graft function. Transplantation 2015, 99, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.E.; Doshi, M.D.; Poggio, E.D.; Parikh, C.R. A comparison of alternative serum biomarkers with creatinine for predicting allograft function after kidney transplantation. Transplantation 2011, 91, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A.; Reilly, E.S. Quantifying glomerular filtration rates in acute kidney injury: A requirement for translational success. Semin. Nephrol. 2016, 36, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J. Am. Soc. Nephrol. 2013, 24, 877–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, E.D.; Doyle, A. The clinical utility of kinetic glomerular filtration rate. Clin. Kidney J. 2016, sfw108. [Google Scholar] [CrossRef] [Green Version]
- Dewitte, A.; Joannès-Boyau, O.; Sidobre, C.; Fleureau, C.; Bats, M.-L.; Derache, P.; Leuillet, S.; Ripoche, J.; Combe, C.; Ouattara, A. Kinetic eGFR and Novel AKI Biomarkers to Predict Renal Recovery. Clin. J. Am. Soc. Nephrol. 2015, 10, 1900–1910. [Google Scholar] [CrossRef] [Green Version]
- Chen, S. Kinetic glomerular filtration rate in routine clinical practice-applications and possibilities. Adv. Chronic Kidney Dis. 2018, 25, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Pianta, T.J.; Endre, Z.H.; Pickering, J.W.; Buckley, N.A.; Peake, P.W. Kinetic estimation of GFR improves prediction of dialysis and recovery after kidney transplantation. PLoS ONE 2015, 10, e0125669. [Google Scholar] [CrossRef]
- Vilar, E.; Varagunam, M.; Yaqoob, M.; Raftery, M.; Thuraisingham, R. Creatinine reduction ratio: A useful marker to identify medium and high-risk renal transplants. Transplantation 2010, 89, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, A.A.; Naghibi, M.; Kianoush, S.; Kianoosh, S.; Ashraf, H. Impact of slow and delayed graft function on kidney graft survival between various subgroups among renal transplant patients. Transplant. Proc. 2009, 41, 2777–2780. [Google Scholar] [CrossRef] [PubMed]
- Nel, D.; Vogel, J.; Muller, E.; Barday, Z.; Kahn, D. Slow early graft function: A neglected entity after renal transplantation. Nephron Clin. Pract. 2012, 120, c200–c204. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Jani, A.; Mishra, J.; Ma, Q.; Kelly, C.; Barasch, J.; Edelstein, C.L.; Devarajan, P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am. J. Transplant. 2006, 6, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, H.M.; Seo, M.Y.; Lee, J.Y.; Kim, K.; Jun, H.; Jung, C.W.; Park, K.T.; Kim, M.-G.; Jo, S.-K.; et al. Urine liver-type fatty acid-binding protein predicts graft outcome up to 2 years after kidney transplantation. Transplant. Proc. 2014, 46, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Poniatowski, B.; Malyszko, J.S.; Dobrzycki, S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren. Fail. 2009, 31, 910–919. [Google Scholar] [CrossRef]
- Seelhammer, T.G.; Maile, M.D.; Heung, M.; Haft, J.W.; Jewell, E.S.; Engoren, M. Kinetic estimated glomerular filtration rate and acute kidney injury in cardiac surgery patients. J. Crit. Care 2016, 31, 249–254. [Google Scholar] [CrossRef]
- Yoshida, M.; Matsuyama, Y.; Ohashi, Y. Estimation of treatment effect adjusting for dependent censoring using the IPCW method: An application to a large primary prevention study for coronary events (MEGA study). Clin. Trials 2007, 4, 318–328. [Google Scholar] [CrossRef]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Li, L.; Beck, G.J.; Joffe, M.M.; Froissart, M.; Kusek, J.W.; Zhang, Y.; Coresh, J.; et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009, 75, 652–660. [Google Scholar] [CrossRef] [Green Version]

| No SGF (n = 250) | SGF (n = 76) | Total (n = 326) | p-Value | |
|---|---|---|---|---|
| Age, median (Q1, Q3) | 51.50 (42.00, 62.00) | 58.00 (44.75, 68.00) | 53.00 (43.00, 63.00) | 0.009 |
| Male sex | 151 (60.4%) | 48 (63.2%) | 199 (61.0%) | 0.692 |
| Pre-emptive transplantation | 37 (14.8%) | 4 (5.3%) | 41 (12.6%) | 0.030 |
| ABO incompatible | 21 (7.9%) | 3 (3.8%) | 24 (6.9%) | 0.223 |
| Living donor | 119 (47.6%) | 12 (15.8%) | 131 (40.2%) | <0.001 |
| Donation after cardiac death | 3 (1.2%) | 8 (10.5%) | 11 (3.4%) | <0.001 |
| Donor age, median (Q1, Q3) | 52.00 (43.00, 61.00) | 57.00 (45.50, 68.50) | 53.00 (43.00, 62.50) | 0.017 |
| Dialysis | 2 (0.8%) | 5 (6.6%) | 7 (9.6%) | 0.003 |
| 3 month eGFR, median (Q1, Q3) | 56.12 (47.54, 67.66) | 41.75 (32.04, 62.65) | 54.02 (43.89, 67.32) | <0.001 |
| 6 month eGFR, median (Q1, Q3) | 54.23 (45.47, 68.59) | 46.04 (30.99, 59.97) | 53.22 (42.46, 67.69) | <0.001 |
| 1 year eGFR, median (Q1, Q3) | 56.93 (45.30, 68.57) | 46.92 (37.99, 59.90) | 53.58 (44.35, 67.73) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, J.; Verissimo, T.; Faivre, A.; Berchtold, L.; Berney, T.; Pugin, J.; de Seigneux, S.; Legouis, D. Kinetic GFR Outperforms CKD-EPI for Slow Graft Function Prediction in the Immediate Postoperative Period Following Kidney Transplantation. J. Clin. Med. 2020, 9, 4003. https://doi.org/10.3390/jcm9124003
Dash J, Verissimo T, Faivre A, Berchtold L, Berney T, Pugin J, de Seigneux S, Legouis D. Kinetic GFR Outperforms CKD-EPI for Slow Graft Function Prediction in the Immediate Postoperative Period Following Kidney Transplantation. Journal of Clinical Medicine. 2020; 9(12):4003. https://doi.org/10.3390/jcm9124003
Chicago/Turabian StyleDash, Jonathan, Thomas Verissimo, Anna Faivre, Lena Berchtold, Thierry Berney, Jérôme Pugin, Sophie de Seigneux, and David Legouis. 2020. "Kinetic GFR Outperforms CKD-EPI for Slow Graft Function Prediction in the Immediate Postoperative Period Following Kidney Transplantation" Journal of Clinical Medicine 9, no. 12: 4003. https://doi.org/10.3390/jcm9124003





