The Implementation of a Complication Avoidance Care Bundle Significantly Reduces Adverse Surgical Outcomes in Orthopedic Trauma Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Complication Avoidance Care Bundle
2.1.1. Improving Team Situational Awareness
2.1.2. Reducing Operating Room Traffic by Staff Members and Limiting Door-Opening Events
2.1.3. Preoperative Screening for Infectious Foci (Including Urinary Tract Infection)
2.1.4. Adapted Preoperative Antibiotic Prophylaxis in Anatomic Regions with a High Risk of Infectious Complications
2.1.5. Use of Iodine-Impregnated Adhesive Drape
2.2. Statistical Analysis
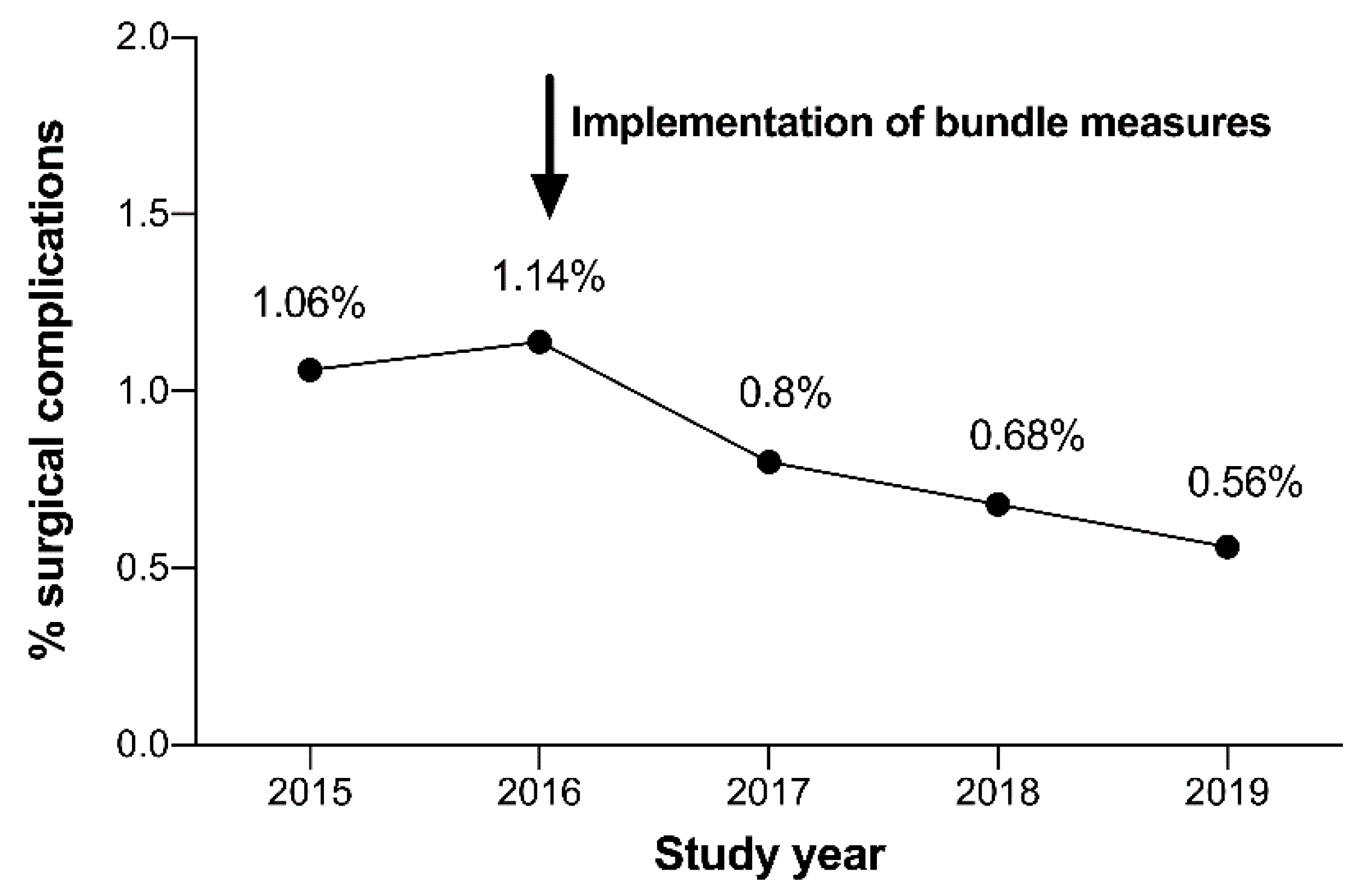
3. Results
Complications before and after the Implementation of the “Complication Avoidance Care Bundle”
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Vries, E.N.; Ramrattan, D.A.; Smorenburg, S.M.; Gouma, D.J.; Boermeester, M.A. The incidence and nature of in-hospital adverse events: A systematic review. Qual. Saf. Health Care 2008, 17, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurusamy, K.; Aggarwal, R.; Palanivelu, L.; Davidson, B.R. Systematic review of randomized controlled trials on the effectiveness of virtual reality training for laparoscopic surgery. Br. J. Surg. 2008, 95, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Mishra, A.; Handa, A.; Dale, T.; Hirst, G.; Catchpole, K. The effects of aviation-style non-technical skills training on technical performance and outcome in the operating theatre. Qual. Saf. Health Care 2009, 18, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloystein, D.M.; Heiges, B.A.; Schwartz, D.G.; DeVine, J.G.; Spratt, D. Innovative Technology System to Prevent Wrong Site Surgery and Capture Near Misses: A Multi-Center Review of 487 Cases. Front. Surg. 2020, 7, 563337. [Google Scholar] [CrossRef]
- Angle, J.F.; Nemcek, A.A., Jr.; Cohen, A.M.; Miller, D.L.; Grassi, C.J.; D’Agostino, H.R.; Khan, A.A.; Kundu, S.; Osnis, R.B.; Rajan, D.K.; et al. Quality improvement guidelines for preventing wrong site, wrong procedure, and wrong person errors: Application of the joint commission “universal protocol for preventing wrong site, wrong procedure, wrong person surgery” to the practice of interventional radiology. J. Vasc. Interv. Radiol. 2009, 20, S256–S262. [Google Scholar] [CrossRef]
- Haynes, A.B.; Weiser, T.G.; Berry, W.R.; Lipsitz, S.R.; Breizat, A.H.; Dellinger, E.P.; Herbosa, T.; Joseph, S.; Kibatala, P.L.; Lapitan, M.C.; et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N. Engl. J. Med. 2009, 360, 491–499. [Google Scholar] [CrossRef]
- Lingard, L.; Regehr, G.; Orser, B.; Reznick, R.; Baker, G.R.; Doran, D.; Espin, S.; Bohnen, J.; Whyte, S. Evaluation of a preoperative checklist and teAm. briefing among surgeons, nurses, and anesthesiologists to reduce failuRes. in communication. Arch. Surg. 2008, 143, 12–17, discussion 18. [Google Scholar] [CrossRef] [Green Version]
- Santiesteban, L.; Hutzler, L.; Bosco, J.A., 3rd; Robb, W., 3rd. Wrong-Site Surgery in Orthopaedics: Prevalence, Risk Factors, and Strategies for Prevention. JBJS Rev. 2016, 4. [Google Scholar] [CrossRef]
- de Vries, E.N.; Prins, H.A.; Crolla, R.M.; den Outer, A.J.; van Andel, G.; van Helden, S.H.; Schlack, W.S.; van Putten, M.A.; Gouma, D.J.; Dijkgraaf, M.G.; et al. Effect of a comprehensive surgical safety system on patient outcomes. N. Engl. J. Med. 2010, 363, 1928–1937. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, D.N.; Vincent, L.E.; Pearson, N.; Beaven, A.; Smith, I.M.; Smith, K.; Toman, E.; Dorrance, H.R.; Porter, K.; Wade, C.E.; et al. An adapted Clavien-Dindo scoring system in trauma as a clinically meaningful nonmortality endpoint. J. Trauma Acute Care Surg. 2017, 83, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.E.; Jurkovich, G.J.; Galante, J.M.; Farmer, D.L. A Survey of the Surgical Morbidity and Mortality Conference in the United States and Canada: A Dying Tradition or the Key to Modern Quality Improvement? J. Surg. Educ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, J.M.; Mourany, J.; Afridi, F.G.; Musgrove, K.; Shaffer, L.; Khan, U.; Marsh, J.W.; Borgstrom, D.C. Enhancing the Educational Value and Faculty Attendance of a Morbidity and Mortality Conference. J. Surg. Educ. 2020, 77, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Harbison, S.P.; Regehr, G. Faculty and resident opinions regarding the role of morbidity and mortality conference. Am. J. Surg. 1999, 177, 136–139. [Google Scholar] [CrossRef]
- Deis, J.N.; Smith, K.M.; Warren, M.D.; Throop, P.G.; Hickson, G.B.; Joers, B.J.; Deshpande, J.K. Transforming the Morbidity and Mortality Conference into an Instrument for Systemwide Improvement. In Advances in Patient Safety: New Directions and Alternative Approaches (Volume 2: Culture and Redesign); Henriksen, K., Battles, J.B., Keyes, M.A., Grady, M.L., Eds.; AHRQ Publication: Rockville, MD, USA, 2008. [Google Scholar]
- Wagner, G.; Gritzbach, B.; Frank, J.; Marzi, I. A transparent, internal complication management concept: Results and consequences. Z. Orthop. Unf. 2010, 148, 520–524. [Google Scholar] [CrossRef]
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections: The new CDC guidelines. JAAPA 2018, 31, 52–54. [Google Scholar] [CrossRef]
- Marche, B.; Neuwirth, M.; Kugler, C.; Bouillon, B.; Mattner, F.; Otchwemah, R. Implementation methods of infection prevention measuRes. in orthopedics and traumatology—A systematic review. Eur. J. Trauma Emerg. Surg. 2020. [Google Scholar] [CrossRef]
- Uckay, I.; Hoffmeyer, P.; Lew, D.; Pittet, D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: State-of-the-art update. J. Hosp. Infect. 2013, 84, 5–12. [Google Scholar] [CrossRef]
- Dobson, P.F.; Reed, M.R. Prevention of infection in primary THA and TKA. EFORT Open Rev. 2020, 5, 604–613. [Google Scholar] [CrossRef]
- Graafland, M.; Schraagen, J.M.; Boermeester, M.A.; Bemelman, W.A.; Schijven, M.P. Training situational awareness to reduce surgical errors in the operating room. Br. J. Surg. 2015, 102, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabri, P.J.; Zayas-Castro, J.L. Human error, not communication and systems, underlies surgical complications. Surgery 2008, 144, 557–563, discussion 563-555. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.O., Jr.; Gawande, A.A.; Kwaan, M.; Puopolo, A.L.; Yoon, C.; Brennan, T.A.; Studdert, D.M. Analysis of surgical errors in closed malpractice claims at 4 liability insurers. Surgery 2006, 140, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.C.; Moucha, C.S. Operating-Room Airflow Technology and Infection Prevention. J. Bone Jt. Surg. Am. 2018, 100, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Salassa, T.E.; Swiontkowski, M.F. Surgical attire and the operating room: Role in infection prevention. J. Bone Jt. Surg. Am. 2014, 96, 1485–1492. [Google Scholar] [CrossRef] [Green Version]
- Bozic, K.J.; Lau, E.; Kurtz, S.; Ong, K.; Berry, D.J. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin. Orthop. Relat. Res. 2012, 470, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.; Munoz-Mahamud, E.; Quayle, J.; Dias da Costa, L.; Casals, C.; Scott, P.; Leite, P.; Vilanova, P.; Garcia, S.; Ramos, M.H.; et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin. Infect. Dis 2014, 59, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Ampuero, J.; Gonzalez-Fernandez, E.; Martinez-Velez, D.; Esteban, J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin. Orthop. Relat. Res. 2013, 471, 3822–3829. [Google Scholar] [CrossRef] [Green Version]
- Yassa, R.R.; Khalfaoui, M.Y.; Veravalli, K.; Evans, D.A. Pre-operative urinary tract infection: Is it a risk factor for early surgical site infection with hip fracture surgery? A retrospective analysis. JRSM Open 2017, 8, 2054270416675083. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [Green Version]
- Husted, H.; Gromov, K.; Malchau, H.; Freiberg, A.; Gebuhr, P.; Troelsen, A. Traditions and myths in hip and knee arthroplasty. Acta Orthop. 2014, 85, 548–555. [Google Scholar] [CrossRef]
- Kramer, A.; Assadian, O.; Lademann, J. Prevention of postoperative wound infections by covering the surgical field with iodine-impregnated incision drape (Ioban 2). GMS Krankenhhyg Interdiszip 2010, 5. [Google Scholar] [CrossRef]
- Webster, J.; Alghamdi, A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst. Rev. 2015, CD006353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, M.A.; Campbell, E.D. Retrospective evaluation of an iodophor-incorporated antimicrobial plastic adhesive wound drape. Clin. Orthop. Relat. Res. 1988, 228, 307–308. [Google Scholar] [CrossRef]
- AlBuhairan, B.; Hind, D.; Hutchinson, A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: A systematic review. J. Bone Jt. Surg. Br. 2008, 90, 915–919. [Google Scholar] [CrossRef] [PubMed]


| All Years | 2015–2016 | 2017–2019 | p-Value | |
|---|---|---|---|---|
| Patients admitted to hospital | 16,349 | 6677 | 9672 | |
| Total number of surgical procedures | 19,096 | 7448 | 11,648 | |
| All surgical complications, % (n) | 0.84% (161) | 1.10% (82) | 0.68% (79) | 0.002 |
| Surgical site infection (SSI), % (n) | 0.21% (40) | 0.32% (24) | 0.14% (16) | 0.006 |
| Early postoperative dislocation after arthroplasty, % (n) * | 0.09% (18) | 0.11% (8) | 0.09% (10) | 0.636 |
| Periprosthetic fracture, % (n) | 0.05% (10) | 0.08% (6) | 0.03% (4) | 0.173 |
| Screw malposition, % (n) ** | 0.1% (20) | 0.15% (11) | 0.08% (9) | 0.142 |
| Suboptimal fracture reduction, % (n) | 0.09% (18) | 0.13% (10) | 0.07% (8) | 0.150 |
| Postoperative neurological deficit, % (n) *** | 0.06% (12) | 0.09% (7) | 0.04% (5) | 0.170 |
| Intraoperative bleeding complication, % (n) | 0.05% (9) | 0.07% (5) | 0.03% (4) | 0.309 |
| Various, % (n) | 0.18% (34) | 0.15% (11) | 0.2% (23) | 0.426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lustenberger, T.; Meier, S.L.; Verboket, R.D.; Störmann, P.; Janko, M.; Frank, J.; Marzi, I. The Implementation of a Complication Avoidance Care Bundle Significantly Reduces Adverse Surgical Outcomes in Orthopedic Trauma Patients. J. Clin. Med. 2020, 9, 4006. https://doi.org/10.3390/jcm9124006
Lustenberger T, Meier SL, Verboket RD, Störmann P, Janko M, Frank J, Marzi I. The Implementation of a Complication Avoidance Care Bundle Significantly Reduces Adverse Surgical Outcomes in Orthopedic Trauma Patients. Journal of Clinical Medicine. 2020; 9(12):4006. https://doi.org/10.3390/jcm9124006
Chicago/Turabian StyleLustenberger, Thomas, Simon Lars Meier, René Danilo Verboket, Philipp Störmann, Maren Janko, Johannes Frank, and Ingo Marzi. 2020. "The Implementation of a Complication Avoidance Care Bundle Significantly Reduces Adverse Surgical Outcomes in Orthopedic Trauma Patients" Journal of Clinical Medicine 9, no. 12: 4006. https://doi.org/10.3390/jcm9124006





