Canaloplasty in Pigmentary Glaucoma: Long-Term Outcomes and Proposal of a New Hypothesis on Its Intraocular Pressure Lowering Mechanism
Abstract
:1. Introduction
2. Study Design
3. Material and Methods
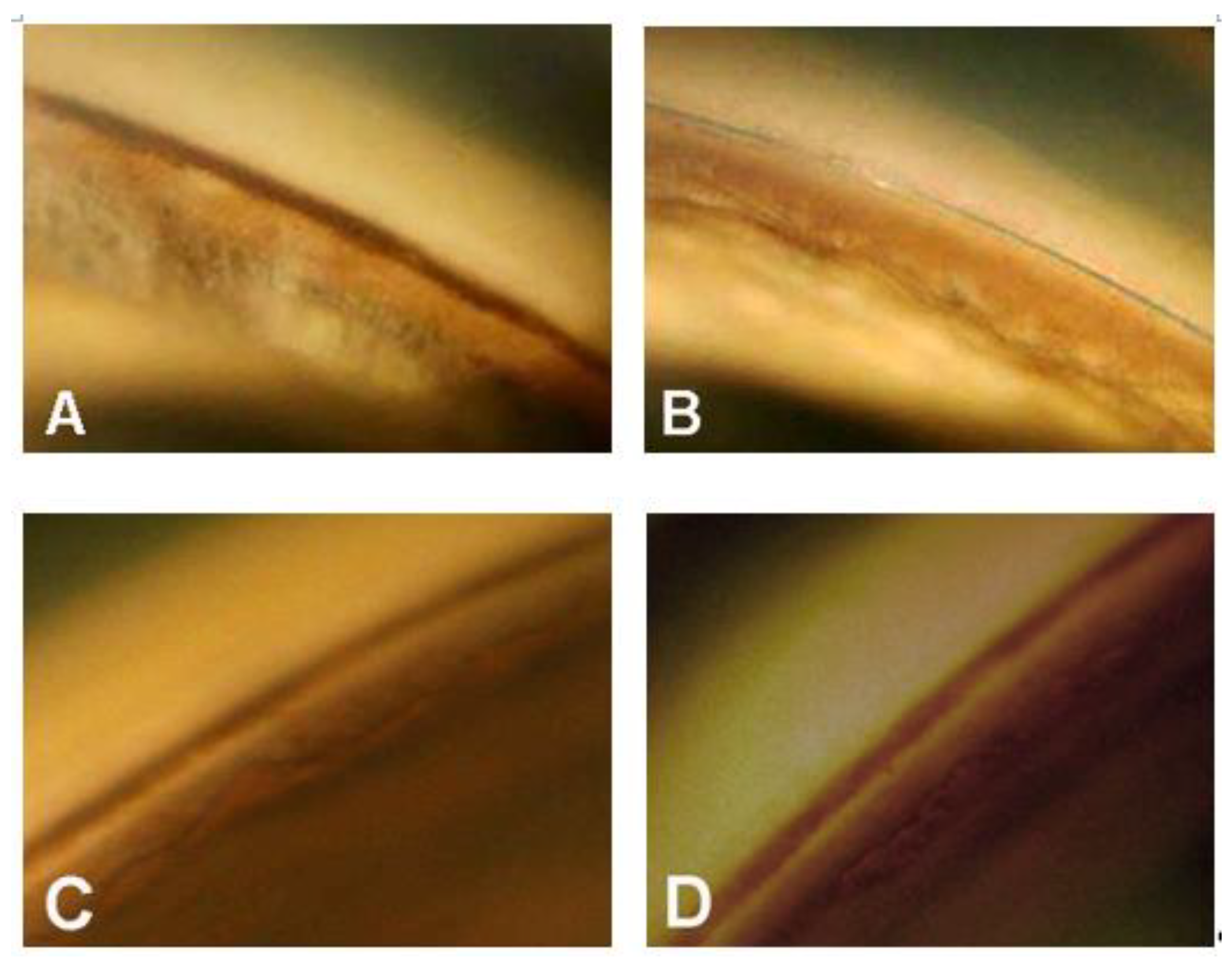
4. Results
- In three eyes, the procedure was converted into a viscocanalostomy (viscodilation of Schlemm’s canal without suture positioning), due to the impossibility of completing the 360° cannulation. These three eyes were excluded from statistical analysis. Four patients underwent a trabeculectomy after 3 to 30 months due to an uncontrolled IOP. The last IOP value before trabeculectomy was considered for statistical analysis.
- The follow-up ranged between 19 and 137 months (mean 59.8 ± 30.1), excluding the four eyes that underwent trabeculectomy).
- The pre-operative mean IOP was 31.8 mmHg ± 10.9 (range 21–70) with an average of 3.3 medications. The IOP values along various follow-up periods are shown in Figure 1.
- After 1, 2, 3, and 4 years, the mean IOP was 15.9 ± 4.0, 14.4 ± 7.3, 14.1 ± 2.1, and 15.7 ± 6.8 mmHg, respectively, with 0.4, 0.5, 0.6, and 1.1 medications, respectively (Figure 2).
- The complete and qualified (in brackets) success rate—defined as an IOP ≤ 21 mmHg—after 1, 2, 3, and 4 years was 80.0% (96.0), 57.9% (84.2), 62.3% (92.9), and 50% (87.5), respectively. Considering an IOP ≤ 18 mmHg as the criterion for success, the percent of success in our surgical patients over 4 years were: 68.0% (84.0), 57.9% (84.2), 57.1% (85.7), and 50% (87.7), respectively. Visual field damage showed no significant progression in all patients during the follow-up period.
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheie, H.G.; Cameron, J.D. Pigment dispersion syndrome: A clinical study. Br. J. Ophthalmol. 1981, 65, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyadurupola, N.; Broadway, D.C. Pigment dispersion syndrome and pigmentary glaucoma—A major review. Clin. Exp. Ophthalmol. 2008, 36, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.G. Pigmentary dispersion and glaucoma: A new theory. Arch. Ophthalmol. 1979, 97, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.G.; Schertzer, R.M. Pathophysiology of pigment dispersion syndrome and pigmentary glaucoma. Curr. Opin. Ophthalmol. 1995, 6, 96–101. [Google Scholar] [CrossRef]
- Speakman, J.S. Pigmentary dispersion. Br. J. Ophthalmol. 1981, 65, 249–251. [Google Scholar] [CrossRef]
- Ritch, R. Nonprogressive Low-Tension Glaucoma with Pigmentary Dispersion. Am. J. Ophthalmol. 1982, 94, 190–196. [Google Scholar] [CrossRef]
- Ritch, R. Pigmentary glaucoma: A self-limiting entity? Ann. Ophthalmol. 1983, 15, 115–116. [Google Scholar]
- Migliazzo, C.V.; Shaffer, R.N.; Nykin, R.; Magee, S. Long-term analysis of pigmentary dispersion syndrome and pigmentary glaucoma. Ophthalmology 1986, 93, 1528–1536. [Google Scholar] [CrossRef]
- Richter, C.U.; Richardson, T.M.; Grant, W.M. Pigmentary dispersion syndrome and pigmentary glaucoma. A prospective study of the natural history. Arch. Ophthalmol. 1986, 104, 211–215. [Google Scholar] [CrossRef]
- Lehto, I. Long-term prognosis of pigmentary glaucoma. Acta Ophthalmol. 2009, 69, 437–443. [Google Scholar] [CrossRef]
- Okafor, K.; Vinod, K.; Gedde, S.J. Update on pigment dispersion syndrome and pigmentary glaucoma. Curr. Opin. Ophthalmol. 2017, 28, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.; Contestabile, M.T.; Scuderi, L.; Librando, A.; Fenicia, V.; Rahimi, S. Pigment dispersion syndrome and pigmentary glaucoma: A review and update. Int. Ophthalmol. 2019, 39, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.P.; Wang, N.L.; Wang, T.; Chen, H.; Mou, D.P. Long-term Efficacy of Trabeculectomy on Chinese Patients with Pigmentary Glaucoma: A Prospective Case Series Observational Study. Chin. Med. J. 2016, 129, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, P.C.; Dietlein, T.S.; Krieglstein, G.K. Effect of trabecular aspiration on intraocular pressure in pigment dispersion syndrome and pigmentary glaucoma. Ophthalmology 2000, 107, 417–421. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Higashide, T.; Sugiyama, K. Case of pigmentary glaucoma treated with medical therapy, laser treatment, and trabeculotomy. Nippon. Ganka Gakkai Zasshi 2007, 111, 95–101. [Google Scholar] [PubMed]
- Akil, H.; Chopra, V.; Huang, A.; Loewen, N.; Noguchi, J.; Francis, B.A. Clinical results of ab interno trabeculotomy using the Trabectome in patients with pigmentary glaucoma compared to primary open angle glaucoma. Clin. Exp. Ophthalmol. 2016, 44, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.J.; Ibach, M.; Schweitzer, J.; Karpuk, K.L.; Stephens, J.D.; Berdahl, J.P. Trabecular micro-bypass stent implantation with cataract extraction in pigmentary glaucoma. Clin. Exp. Ophthalmol. 2020, 48, 37–43. [Google Scholar] [CrossRef]
- Lavia, C.; Dallorto, L.; Maule, M.; Ceccarelli, M.; Fea, A.M. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0183142. [Google Scholar] [CrossRef]
- Lewis, R.A.; Von Wolff, K.; Tetz, M.; Koerber, N.; Kearney, J.R.; Shingleton, B.J.; Samuelson, T.W. Canaloplasty: Three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J. Cataract. Refract. Surg. 2011, 37, 682–690. [Google Scholar] [CrossRef]
- Bull, H.; Von Wolff, K.; Körber, N.; Tetz, M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1537–1545. [Google Scholar] [CrossRef]
- Brusini, P.; Caramello, G.; Benedetti, S.; Tosoni, C. Canaloplasty in open-angle glaucoma. Mid-term results from a multicenter study. J. Glaucoma 2016, 25, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Brusini, P. Canaloplasty in open-angle glaucoma surgery: A four-year follow-up. Sci. World J. 2014, 2014, 469609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieshaber, M.C.; Pienaar, A.; Olivier, J.; Stegmann, R. Canaloplasty for open-angle glaucoma: Long term outcome. Br. J. Ophthalmol. 2010, 94, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, A.; Müller, M. Trabeculectomy versus canaloplasty. Utility and cost-effectiveness analysis. Klin. Mbl. Augenheilkd. 2012, 229, 1118–1123. [Google Scholar]
- Ayyala, R.S.; Chaudhry, A.L.; Okogbaa, C.B.; Zurakowski, D. Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months’ follow-Up. Ophthalmology 2011, 118, 2427–2433. [Google Scholar] [CrossRef]
- Matlach, J.; Dhillon, C.; Hain, J.; Schlunck, G.; Grehn, F.; Klink, T. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: A prospective randomized clinical trial. Acta Ophthalmol. 2015, 93, 753–761. [Google Scholar] [CrossRef]
- Gabai, A.; Cimarosti, R.; Battistella, C.; Isola, M.; Lanzetta, P. Efficacy and safety of trabeculectomy versus nonpenetrating surgeries in open-angle glaucoma: A Meta-analysis. J. Glaucoma 2019, 28, 823–833. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Pienaar, A.; Olivier, J.; Stegmann, R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Investig. Opthalmol. Vis. Sci. 2010, 51, 1498–1504. [Google Scholar] [CrossRef] [Green Version]
- Brusini, P.; Filacorda, S. Enhanced glaucoma staging system (GSS 2) for classifying functional damage in glaucoma. J. Glaucoma 2006, 15, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Lehto, I. Long-term follow up of argon laser trabeculoplasty in pigmentary glaucoma. Ophthalmic Surg. 1992, 23, 614–617. [Google Scholar]
- Ritch, R.; Liebmann, J.; Robin, A.L.; Pollack, I.P.; Harrison, R.; Levene, R.Z.; Hagadus, J. Argon laser trabeculoplasty in pigmentary glaucoma. Ophthalmology 1993, 100, 909–913. [Google Scholar] [CrossRef]
- Ayala, M. Long-term outcomes of selective laser trabeculoplasty (SLT) treatment in pigmentary glaucoma patients. J. Glaucoma 2014, 23, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Ichhpujani, P.; Barahimi, B.; Shields, C.L.; Eagle, R.C.; Katz, L.J. Canaloplasty for pigmentary glaucoma with coexisting conjunctival lymphoma. Ophthalmic Surg. Lasers Imaging 2011, 42, e10–e11. [Google Scholar] [PubMed]
- Murphy, C.G.; Johnson, M.; Alvarado, J.A. Juxtacanalicular tissue in pigmentary and primary open angle glaucoma. The hydrodynamic role of pigment and other constituents. Arch. Ophthalmol. 1992, 110, 1779–1785. [Google Scholar] [CrossRef]
- Alvarado, J.A.; Murphy, C.G. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992, 110, 1769–1778. [Google Scholar] [CrossRef]
- Gottanka, J.; Johnson, D.H.; Grehn, F.; Lütjen-Drecoll, E. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J. Glaucoma 2006, 15, 142–151. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusini, P.; Papa, V. Canaloplasty in Pigmentary Glaucoma: Long-Term Outcomes and Proposal of a New Hypothesis on Its Intraocular Pressure Lowering Mechanism. J. Clin. Med. 2020, 9, 4024. https://doi.org/10.3390/jcm9124024
Brusini P, Papa V. Canaloplasty in Pigmentary Glaucoma: Long-Term Outcomes and Proposal of a New Hypothesis on Its Intraocular Pressure Lowering Mechanism. Journal of Clinical Medicine. 2020; 9(12):4024. https://doi.org/10.3390/jcm9124024
Chicago/Turabian StyleBrusini, Paolo, and Veronica Papa. 2020. "Canaloplasty in Pigmentary Glaucoma: Long-Term Outcomes and Proposal of a New Hypothesis on Its Intraocular Pressure Lowering Mechanism" Journal of Clinical Medicine 9, no. 12: 4024. https://doi.org/10.3390/jcm9124024




