Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
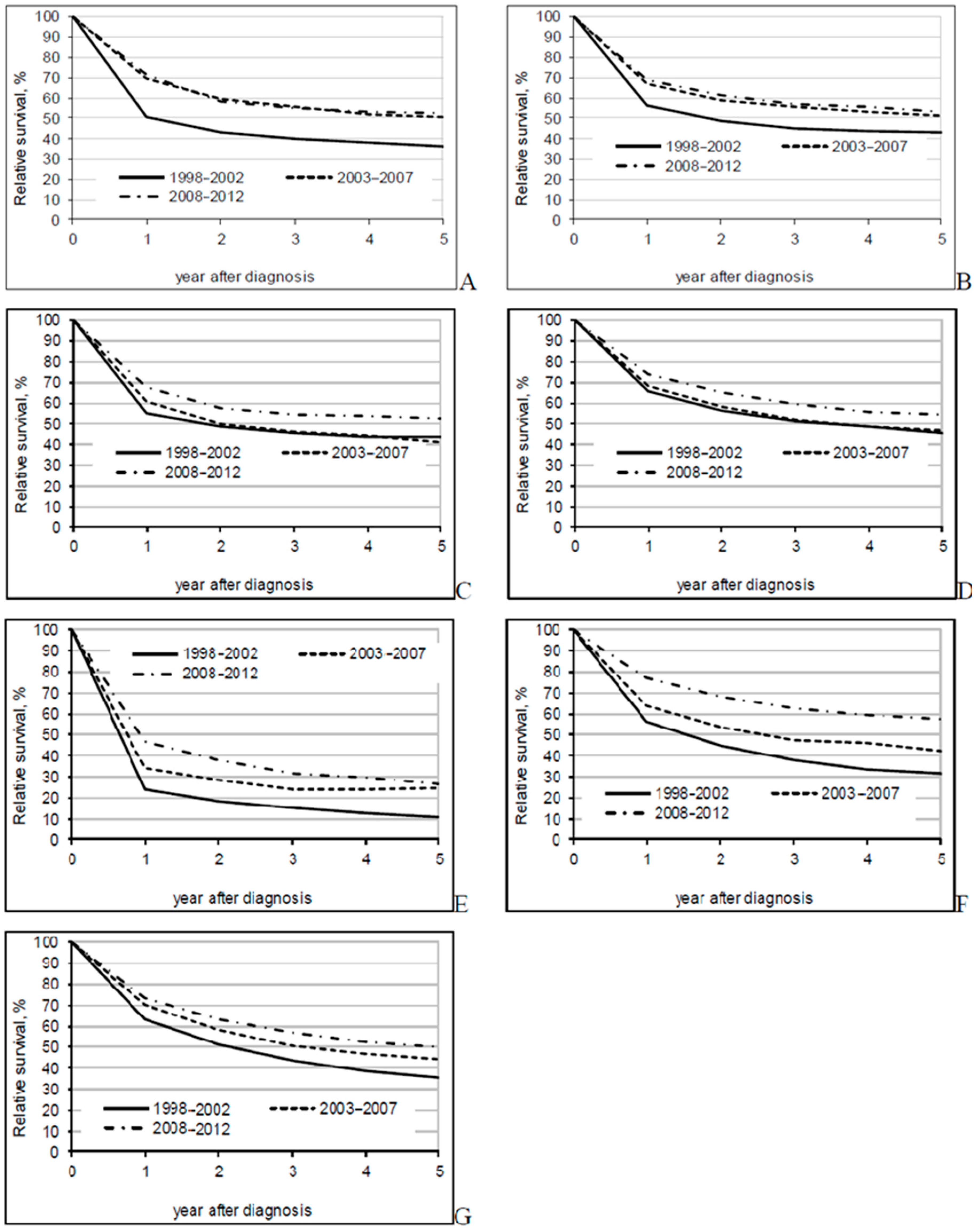
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ouakrim, D.A.; Pizot, C.; Boniol, M.; Malvezzi, M.; Boniol, M.; Negri, E.; Bota, M.; Jenkis, M.A.; Bielberg, H.; Autier, P. Trends in colorectal cancer mortality in Europe: Retrospective analysis of the WHO mortality database. BMJ 2015, 351, h4970. [Google Scholar] [CrossRef] [Green Version]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today, Lyon, France. 2018. Available online: https://gco.iarc.fr/today (accessed on 15 December 2019).
- Brenner, H.; Bouvier, A.M.; Foschi, R.; Hackl, M.; Larsen, I.K.; Lemmens, V.; Mangone, L.; Francisci, S.; The EUROCARE Working Group. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int. J. Cancer 2012, 131, 1649–1658. [Google Scholar] [CrossRef]
- Berrino, F.; Verdecchia, A.; Lutz, J.M.; Lombardo, C.; Micheli, A.; Capocaccia, R.; The EUROCARE Working Group. Comparative cancer survival information in Europe. Eur. J. Cancer 2009, 45, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Laversanne, M.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Piñeros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Antoni, S.; et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 2015, 137, 2060–2071. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) The TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Brenner, H.; Gefeller, O.; Hakulinen, T. Period analysis for providing more up-to-date cancer survival data: Theory, empirical evaluation, computational realization and applications. Eur. J. Cancer 2004, 40, 326–335. [Google Scholar] [CrossRef]
- Dickman, P.W. Estimating and Modelling Relative Survival Using Stata. Available online: http://www.pauldickman.com/rsmodel/stata_colon/ (accessed on 15 January 2020).
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Murauskiene, L.; Janoniene, R.; Veniute, M.; van Ginneken, E.; Karanikolos, M. Lithuania: Health system review. Health Syst. Transit. 2013, 15, 1–150. [Google Scholar]
- Kodeda, K.; Johansson, R.; Zar, N.; Birgisson, H.; Dahlberg, M.; Skullman, S.; Lindmark, G.; Glimelius, B.; Påhlman, L.; Martling, A. Time trends, improvements and national auditing of rectal cancer management over an 18- year period. Colorectal Dis. 2015, 17, 168–179. [Google Scholar] [CrossRef]
- Guren, M.G.; Kørner, H.; Pfeffer, F.; Myklebust, T.Å.; Eriksen, M.T.; Edna, T.-H.; Larsen, S.G.; Knudsen, K.O.; Nesbakken, A.; Wasmuth, H.H.; et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993–2010. Acta Oncol. 2015, 54, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Borowski, D.W.; Bradburn, D.M.; Mills, S.J.; Bharathan, B.; Wilson, R.G.; Ratcliffe, A.A.; Kelly, S.B. Volume-outcome analysis of colorectal cancer-related outcomes. Br. J. Surg. 2010, 97, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- van Steenbergen, L.N.; Elferink, M.A.; Krijnen, P.; Lemmens, V.E.P.P.; Siesling, S.; Rutten, H.J.T.; Richel, D.J.; Karim-Kos, H.E.; Coebergh, J.W.W.; on the behalf of the Working Group Output of The Netherlands Cancer Registry. Improved survival of colon cancer due to improved treatment and detection: A nationwide population-based study in The Netherlands 1989–2006. Ann. Oncol. 2010, 21, 2206–2212. [Google Scholar] [CrossRef]
- Andre, T.; Boni, C.; Navarro, M.; Tabereno, M.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [Green Version]
- Andre, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: Updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Glimelius, B.; Grönberg, H.; Järhult, J.; Wallgren, A.; Cavallin-ståhl, E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol. 2003, 42, 476–492. [Google Scholar] [CrossRef]
- Braendengen, M.; Tveit, K.M.; Berglund, Å.; Birkemeyer, E.; Frykholm, G.; Påhlman, L.; Wiig, J.N.; Byström, P.; Bujko, K.; Glimelius, B. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J. Clin. Oncol. 2008, 26, 3687–3694. [Google Scholar] [CrossRef]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, F.; Buchwald, P.; Elmståhl, S.; Syk, I. Wide excision in rightsided colon cancer is associated with decreased survival. Scand. J. Surg. 2013, 102, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, A.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25, 676, 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Buchwald, P.; Hall, C.; Davidson, C.; Dixon, L.; Dobbs, B.; Robinson, B.; Frizelle, F. Improved survival for rectal cancer compared to colon cancer: The four cohort study. ANZ J. Surg. 2018, 88, E114–E117. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomatram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, H.; Schrotz-King, P.; Holleczek, B.; Katalinic, A.; Hoffmeister, M. Declining Bowel Cancer Incidence and Mortality in Germany. Deutsches Ärzteblatt Int. 2016, 113, 101–106. [Google Scholar]
- Iversen, L.H.; Green, A.; Ingeholm, P.; Østerlind, K.; Gögenur, I. Improved survival of colorectal cancer in Denmark during 2001-2012—The efforts of several national initiatives. Acta Oncol. 2016, 55 (Suppl. 2), 10–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, D.A.; Johnson, C.J.; White, A.; Wu, M.; Coleman, M.P. Rectal cancer survival in the United States by race and stage, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. 24), 5037–5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, N.P.M.; Bos, A.C.R.K.; Lemmens, V.E.P.P.; Tanis, P.J.; Nagtegaal, I.D.; de Wilt, J.H.W.; Verhoeven, R.H.A. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, J.M.; Rosenberg, P.S.; Jemal, A. Colorectal cancer incidence patterns in the United States, 1974-2013. J. Natl. Cancer Inst. 2017, 109, 1–6. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Survival Rates for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 15 December 2019).


| 1998–2002 | 2003–2007 | 2008–2012 | Change *, % | ||||
|---|---|---|---|---|---|---|---|
| Cases | % | Cases | % | Cases | % | ||
| Stage | |||||||
| Localized tumor T1-T4 | 2236 | 33.88 | 2824 | 40.46 | 3106 | 41.97 | 38.91 |
| Tumor with regional spread N+ | 2189 | 33.17 | 1968 | 28.20 | 1947 | 26.31 | −11.06 |
| Advanced cancer with distant spread | 1762 | 26.70 | 1687 | 24.17 | 1489 | 20.12 | −15.49 |
| Missing | 413 | 6.26 | 500 | 7.16 | 859 | 11.61 | 107.99 |
| Subsite | |||||||
| Caecum and appendix (C180, C181) | 503 | 7.62 | 459 | 6.58 | 526 | 7.11 | 4.57 |
| Right colon (C182, C183) | 604 | 9.15 | 685 | 9.82 | 814 | 11.00 | 34.77 |
| Transverse colon (C184) | 368 | 5.58 | 351 | 5.03 | 329 | 4.45 | −10.60 |
| Left colon (C185, C186, C187) | 1741 | 26.38 | 1889 | 27.07 | 2107 | 28.47 | 21.02 |
| Other (C188, C189) | 194 | 2.94 | 288 | 4.13 | 283 | 3.82 | 45.88 |
| Rectosigmoid (C19) | 376 | 5.70 | 450 | 6.45 | 507 | 6.85 | 34.84 |
| Rectum (C20) | 2814 | 42.64 | 2857 | 40.94 | 2835 | 38.31 | 0.75 |
| All cases | 6600 | 100.00 | 6979 | 100.00 | 7401 | 100.00 | 12.14 |
| 1998–2002 | 2003–2007 | 2008–2012 | Change *, % | |
|---|---|---|---|---|
| Stage | ||||
| Tumor localized T1-T4 | 66.07 (63.45–68.62) | 70.84 (68.39–73.23) | 78.60 (76.33–80.80) | 12.53 |
| Advanced tumor with regional spread N+ | 40.25 (37.98–42.54) | 49.24 (46.57–51.89) | 52.36 (49.57–55.14) | 12.11 |
| Advanced cancer with distant spread | 4.53 (3.62–5.60) | 6.61 (5.40–7.99) | 6.77 (5.47–8.25) | 2.24 |
| Missing | 26.74 (22.56–31.16) | 22.36 (18.39–26.66) | 29.02 (25.02–33.21) | 2.28 |
| Subsite | ||||
| Caecum and appendix (C18.0, C18.1) | 35.71 (31.05–40.49) | 50.38 (44.65–56.04) | 52.13 (46.60–57.59) | 16.42 |
| Right colon (C18.2, C18.3) | 43.22 (38.58–47.88) | 51.33 (46.54–56.08) | 52.95 (48.38–57.47) | 9.73 |
| Transverse colon (C18.4) | 43.53 (37.78–49.30) | 41.20 (35.12–47.38) | 52.39 (45.73–58.93) | 8.86 |
| Left colon (C18.5, C18.6, C18.7) | 45.77 (43.01–48.52) | 46.65 (43.92–49.37) | 54.12 (51.32–56.89) | 8.35 |
| Other (C18.8, C18.9) | 10.84 (8.00–14.20) | 24.34 (18.49–30.84) | 26.40 (20.60–32.72) | 15.56 |
| Rectosigmoid (C19) | 31.62 (26.47–37.01) | 42.39 (36.76–48.07) | 57.26 (51.26–63.10) | 25.64 |
| Rectum (C20) | 35.61 (33.62–37.63) | 44.49 (42.23–46.74) | 49.59 (47.32–51.86) | 13.98 |
| All cases | 37.86 (36.54–39.19) | 45.04 (43.60–46.48) | 51.13 (49.67–52.58) | 13.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulskas, A.; Gaizauskas, V.; Kildusiene, I.; Samalavicius, N.E.; Smailyte, G. Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study. J. Clin. Med. 2020, 9, 4038. https://doi.org/10.3390/jcm9124038
Dulskas A, Gaizauskas V, Kildusiene I, Samalavicius NE, Smailyte G. Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study. Journal of Clinical Medicine. 2020; 9(12):4038. https://doi.org/10.3390/jcm9124038
Chicago/Turabian StyleDulskas, Audrius, Vytautas Gaizauskas, Inga Kildusiene, Narimantas Evaldas Samalavicius, and Giedre Smailyte. 2020. "Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study" Journal of Clinical Medicine 9, no. 12: 4038. https://doi.org/10.3390/jcm9124038






