The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease
Abstract
1. Processing and Secretion of Glucagon
2. The Glucagon Receptor as a Target in the Treatment of Type 2 Diabetes, Obesity, and NAFLD
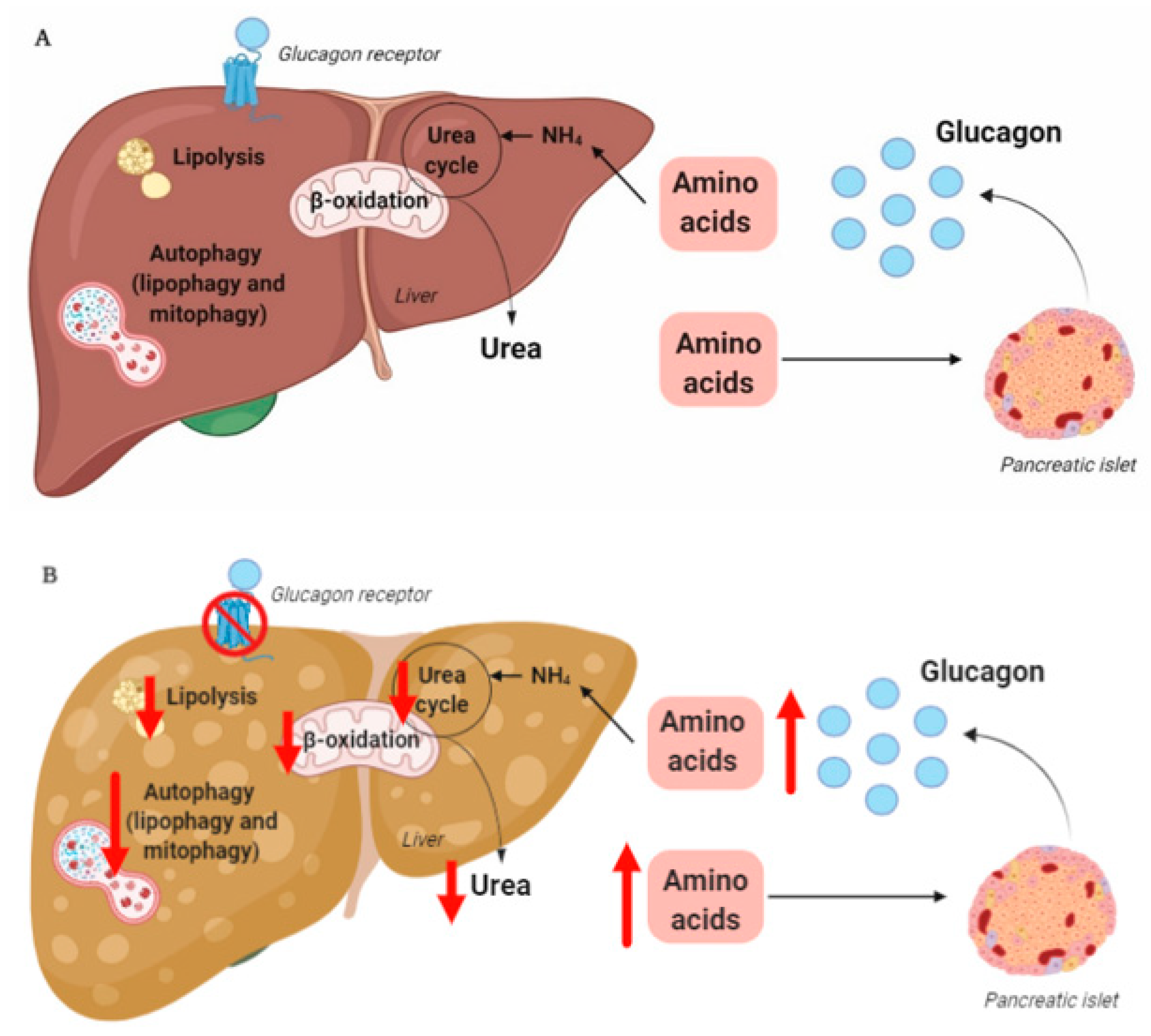
3. The Liver–Alpha Cell Axis May be Impaired in NAFLD
4. Hepatic Glucagon Resistance May Impair Autophagy Resulting in Increased Oxidative Stress
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, G.I.; Sanchez-Pescador, R.; Laybourn, P.J.; Najarian, R.C. Exon duplication and divergence in the human preproglucagon gene. Nature 1983, 304, 368–371. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon and the Glucagon-like Peptides. Pancreas 1990, 5, 484–488. [Google Scholar] [CrossRef]
- Rouille, Y.; Westermark, G.; Martin, S.K.; Steiner, D.F. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1-6 cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3242–3246. [Google Scholar] [CrossRef]
- Rouillé, Y.; Martin, S.; Steiner, D.F. Differential Processing of Proglucagon by the Subtilisin-like Prohormone Convertases PC2 and PC3 to Generate either Glucagon or Glucagon-like Peptide. J. Biol. Chem. 1995, 270, 26488–26496. [Google Scholar] [CrossRef] [PubMed]
- Kilimnik, G.; Kim, A.; Steiner, N.F.; Friedman, T.C.; Hara, M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets 2010, 2, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Whalley, N.M.; Pritchard, L.E.; Smith, D.M.; White, A. Processing of proglucagon to GLP-1 in pancreatic α-cells: Is this a paracrine mechanism enabling GLP-1 to act on β-cells? J. Endocrinol. 2011, 211, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Lupi, R.; Bugliani, M.; Kirkpatrick, C.L.; Sebastiani, G.D.; Grieco, F.A.; Del Guerra, S.; D’Aleo, V.; Piro, S.; Marselli, L.; et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 2012, 55, 3262–3272. [Google Scholar] [CrossRef]
- Taylor, S.W.; Nikoulina, S.E.; Andon, N.L.; Lowe, C. Peptidomic Profiling of Secreted Products from Pancreatic Islet Culture Results in a Higher Yield of Full-length Peptide Hormones than Found using Cell Lysis Procedures. J. Proteome Res. 2013, 12, 3610–3619. [Google Scholar] [CrossRef]
- Holst, J.J.; Pedersen, J.H.; Baldissera, F.; Stadil, F. Circulating glucagon after total pancreatectomy in man. Diabetologia 1983, 25, 396–399. [Google Scholar] [CrossRef]
- Lund, A.; Bagger, J.I.; Albrechtsen, N.J.W.; Christensen, M.; Grøndahl, M.; Hartmann, B.; Mathiesen, E.R.; Hansen, C.P.; Storkholm, J.H.; Van Hall, G.; et al. Evidence of Extrapancreatic Glucagon Secretion in Man. Diabetes 2015, 65, 585–597. [Google Scholar] [CrossRef]
- Jorsal, T.; Albrechtsen, N.J.W.; Christensen, M.M.; Mortensen, B.; Wandall, E.; Langholz, E.; Friis, S.; Worm, D.; Ørskov, C.; Støving, R.K.; et al. Investigating Intestinal Glucagon After Roux-en-Y Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2019, 104, 6403–6416. [Google Scholar] [CrossRef] [PubMed]
- Gelling, R.W.; Du, X.Q.; Dichmann, D.S.; Romer, J.; Huang, H.; Cui, L.; Obici, S.; Tang, B.; Holst, J.J.; Fledelius, C.; et al. Lower blood glucose, hyperglucagonemia, and pancreatic cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Nakashima, M.; Brubaker, P.L.; Li, Q.-L.; Perfetti, R.; Jansen, E.; Zambre, Y.; Pipeleers, D.; Friedman, T.C. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J. Clin. Investig. 2000, 105, 955–965. [Google Scholar] [CrossRef]
- Chambers, A.P.; Sorrell, J.E.; Haller, A.; Roelofs, K.; Hutch, C.R.; Kim, K.-S.; Gutierrez-Aguilar, R.; Li, B.; Drucker, D.J.; D’Alessio, D.A.; et al. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Cell Metab. 2017, 25, 927–934.e3. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.A.; Golec, D.P.; Hubert, M.; Johnson, J.; Salamon, N.; Barr, A.; Macdonald, P.E.; Philippaert, K.; Light, P.E. Human islets contain a subpopulation of glucagon-like peptide-1 secreting α cells that is increased in type 2 diabetes. Mol. Metab. 2020, 39, 101014. [Google Scholar] [CrossRef]
- Fava, G.E.; Dong, E.W.; Wu, H. Intra-islet glucagon-like peptide 1. J. Diabetes Its Complicat. 2016, 30, 1651–1658. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Hartmann, B.; Veedfald, S.; Windeløv, J.A.; Plamboeck, A.; Bojsen-Møller, K.N.; Idorn, T.; Feldt-Rasmussen, B.; Knop, F.K.; Vilsbøll, T.; et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: Nonspecific interference or truly elevated levels? Diabetologia 2014, 57, 1919–1926. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Veedfald, S.; Plamboeck, A.; Deacon, C.F.; Hartmann, B.; Knop, F.K.; Vilsboll, T.; Holst, J.J. Inability of Some Commercial Assays to Measure Suppression of Glucagon Secretion. J. Diabetes Res. 2015, 2016, 1–5. [Google Scholar] [CrossRef]
- Roberts, G.P.; Kay, R.G.; Howard, J.; Hardwick, R.H.; Reimann, F.; Gribble, F.M. Gastrectomy with Roux-en-Y reconstruction as a lean model of bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 562–568. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Clemmensen, C.; DiMarchi, R.D.; Tschöp, M.H. The New Biology and Pharmacology of Glucagon. Physiol. Rev. 2017, 97, 721–766. [Google Scholar] [CrossRef]
- Kelly, R.P.; Garhyan, P.; Raddad, E.; Fu, H.; Lim, C.N.; Prince, M.J.; Pinaire, J.A.; Loh, M.T.; Deeg, M.A. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes. Metab. 2015, 17, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kazda, C.M.; Ding, Y.; Kelly, R.P.; Garhyan, P.; Shi, C.; Lim, C.N.; Fu, H.; Watson, D.E.; Lewin, A.J.; Landschulz, W.H.; et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY2409021 in Patients With Type 2 Diabetes: 12- and 24-Week Phase 2 Studies. Diabetes Care 2015, 39, 1241–1249. [Google Scholar] [CrossRef]
- Kazierad, D.J.; Bergman, A.; Tan, B.; Erion, D.M.; Somayaji, V.; Lee, D.S.; Rolph, T. Effects of multiple ascending doses of the glucagon receptor antagonist PF-06291874 in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2016, 18, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Vajda, E.G.; Logan, D.; Lasseter, K.; Armas, D.; Plotkin, D.J.; Pipkin, J.; Li, Y.-X.; Zhou, R.; Klein, D.; Wei, X.; et al. Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD-6972 in healthy subjects and subjects with type 2 diabetes mellitus. Diabetes Obes. Metab. 2016, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.J.; Konkar, A.; Hornigold, D.C.; Trevaskis, J.L.; Jackson, R.; Fredin, M.F.; Jansson-Löfmark, R.; Naylor, J.; Rossi, A.; Bednarek, M.A.; et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016, 18, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cai, X.; Huang, X.; Dai, Y.; Sun, L.; Zhang, B.; Yang, B.; Lin, H.; Huang, W.; Qian, H. A novel glucagon-like peptide-1/glucagon receptor dual agonist exhibits weight-lowering and diabetes-protective effects. Eur. J. Med. Chem. 2017, 138, 1158–1169. [Google Scholar] [CrossRef]
- More, V.R.; Lao, J.; McLaren, D.G.; Cumiskey, A.-M.; Murphy, B.A.; Chen, Y.; Previs, S.; Stout, S.; Patel, R.; Satapati, S.; et al. Glucagon like receptor 1/ glucagon dual agonist acutely enhanced hepatic lipid clearance and suppressed de novo lipogenesis in mice. PLoS ONE 2017, 12, e0186586. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Müller, W.A.; Faloona, G.R.; Aguilar-Parada, E.; Unger, R.H. Abnormal Alpha-Cell Function in Diabetes. Response to carbohydrate and protein ingestion. N. Engl. J. Med. 1970, 283, 109–115. [Google Scholar] [CrossRef]
- Mitrakou, A.; Kelley, D.; Veneman, T.; Jenssen, T.; Pangburn, T.; Reilly, J.; Gerich, J. Contribution of Abnormal Muscle and Liver Glucose Metabolism to Postprandial Hyperglycemia in NIDDM. Diabetes 1990, 39, 1381–1390. [Google Scholar] [CrossRef]
- Reaven, G.M.; Chen, Y.-D.I.; Golay, A.; Swislocki, A.L.M.; Jaspan, J.B. Documentation of Hyperglucagonemia Throughout the Day in Nonobese and Obese Patients with Noninsulin-Dependent Diabetes Mellitus. J. Clin. Endocrinol. Metab. 1987, 64, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Vella, A.; Basu, A.; Basu, R.; Schwenk, W.F.; Rizza, R.A. Lack of Suppression of Glucagon Contributes to Postprandial Hyperglycemia in Subjects with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2000, 85, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Guzman, C.B.; Zhang, X.M.; Liu, R.; Regev, A.; Shankar, S.; Garhyan, P.; Pillai, S.G.; Kazda, C.; Chalasani, N.; Hardy, T. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1521–1528. [Google Scholar] [CrossRef]
- Kazierad, D.J.; Chidsey, K.; Somayaji, V.R.; Bergman, A.J.; Calle, R.A. Efficacy and safety of the glucagon receptor antagonist PF-06291874: A 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes. Metab. 2018, 20, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Geary, N. Pancreatic glucagon signals postprandial satiety. Neurosci. Biobehav. Rev. 1990, 14, 323–338. [Google Scholar] [CrossRef]
- Geary, N.; Kissileff, H.R.; Pi-Sunyer, F.X.; Hinton, V.J. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am. J. Physiol. Integr. Comp. Physiol. 1992, 262, R975–R980. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.W.F.; Salter, J.M.; Best, C.H. Calorigenic Action of Glucagon. Nat. Cell Biol. 1957, 180, 1124. [Google Scholar] [CrossRef]
- Joel, C.D. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J. Biol. Chem. 1966, 241, 814–821. [Google Scholar]
- Doi, K.; Kuroshima, A. Modified metabolic responsiveness to glucagon in cold-acclimated and heat-acclimated rats. Life Sci. 1982, 30, 785–791. [Google Scholar] [CrossRef]
- Nair, K.S. Hyperglucagonemia Increases Resting Metabolic Rate In Man During Insulin Deficiency. J. Clin. Endocrinol. Metab. 1987, 64, 896–901. [Google Scholar] [CrossRef]
- Dicker, A.; Zhao, J.; Cannon, B.; Nedergaard, J. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am. J. Physiol. Content 1998, 275, R1674–R1682. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, J.L.; Kaur, K.D.; Varin, E.M.; Baggio, L.L.; Cao, X.; Mulvihill, E.E.; Stern, J.H.; Campbell, J.E.; Scherer, P.E.; Drucker, D.J. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab. 2019, 22, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pegorier, J.P.; Garcia-Garcia, M.V.; Prip-Buus, C.; Duee, P.H.; Kohl, C.; Girard, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem. J. 1989, 264, 93–100. [Google Scholar] [PubMed]
- Perry, R.J.; Zhang, D.; Guerra, M.T.; Brill, A.L.; Goedeke, L.; Nasiri, A.R.; Rabin-Court, A.; Wang, Y.; Peng, L.; Dufour, S.; et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nat. Cell Biol. 2020, 579, 279–283. [Google Scholar] [CrossRef]
- Prip-Buus, C.; Pegorier, J.P.; Duee, P.H.; Kohl, C.; Girard, J. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Biochem. J. 1990, 269, 409–415. [Google Scholar]
- Heimberg, M.; Weinstein, I.; Kohout, M. The effects of glucagon, dibutyryl cyclic adenosine 3′,5′-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J. Biol. Chem. 1969, 244, 5131–5139. [Google Scholar]
- Eaton, R.P. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J. Lipid Res. 1973, 14, 312–318. [Google Scholar]
- Longuet, C.; Sinclair, E.M.; Maida, A.; Baggio, L.L.; Maziarz, M.; Charron, M.J.; Drucker, D.J. The Glucagon Receptor Is Required for the Adaptive Metabolic Response to Fasting. Cell Metab. 2008, 8, 359–371. [Google Scholar] [CrossRef]
- Dresler, C.M.; Fortner, J.G.; McDermott, K.; Bajorunas, D.R. Metabolic Consequences of (Regional) Total Pancreatectomy. Ann. Surg. 1991, 214, 131–140. [Google Scholar] [CrossRef]
- Liang, Y.; Osborne, M.C.; Monia, B.P.; Bhanot, S.; Gaarde, W.A.; Reed, C.; She, P.; Jetton, T.L.; Demarest, K.T. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004, 53, 410–417. [Google Scholar] [CrossRef]
- Paloyan, E.; Harper, P.V. Glucagon as a regulating factor of plasma lipids. Metabolism 1961, 10, 315–323. [Google Scholar] [PubMed]
- Amatuzio, D.S.; Grande, F.; Wada, S. Effect of glucagon on the serum lipids in essential hyperlipemia and in hypercholesterolemia. Metabolism 1962, 11, 1240–1249. [Google Scholar] [PubMed]
- Penhos, J.C.; Wu, C.H.; Daunas, J.; Reitman, M.; Levine, R.; Levune, R. Effect of Glucagon on the Metabolism of Lipids and on Urea Formation by the Perfused Rat Liver. Diabetes 1966, 15, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Caren, R.; Corbo, L. Glucagon and cholesterol metabolism. Metabolism 1960, 9, 938–945. [Google Scholar] [PubMed]
- Aubry, F.; Marcel, Y.L.; Davignon, J. Effects of glucagon on plasma lipids in different types of primary hyperlipoproteinemia. Metabolism 1974, 23, 225–238. [Google Scholar] [CrossRef]
- Guettet, C.; Mathe, D.; Riottot, M.; Lutton, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim. Biophys. Acta 1988, 963, 215–223. [Google Scholar] [CrossRef]
- Guettet, C.; Rostaqui, N.; Mathe, D.; Lécuyer, B.; Navarro, N.; Jacotot, B. Effect of chronic glucagon administration on lipoprotein composition in normally fed, fasted and cholesterol-fed rats. Lipids 1991, 26, 451–458. [Google Scholar] [CrossRef]
- Gu, W.; Lloyd, D.J.; Chinookswong, N.; Komorowski, R.; Sivits, G.; Graham, M.; Winters, K.A.; Yan, H.; Boros, L.G.; Lindberg, R.A.; et al. Pharmacological Targeting of Glucagon and Glucagon-Like Peptide 1 Receptors Has Different Effects on Energy State and Glucose Homeostasis in Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2011, 338, 70–81. [Google Scholar] [CrossRef]
- Sadry, S.A.; Drucker, D.J. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat. Rev. Endocrinol. 2013, 9, 425–433. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-Like Peptide 1/Glucagon Receptor Dual Agonism Reverses Obesity in Mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.C.; Joharapurkar, A.; Kshirsagar, S.; Sutariya, B.; Patel, M.; Patel, H.; Pandey, D.; Patel, D.; Ranvir, R.; Kadam, S.; et al. Coagonist of GLP-1 and Glucagon Receptor Ameliorates Development of Non-Alcoholic Fatty Liver Disease. Cardiovasc. Hematol. Agents Med. Chem. 2018, 16, 35–43. [Google Scholar] [CrossRef]
- Younossi, Z. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Kim, J.; Lee, Y.; Kim, Y.; Kang, J.; Trautmann, M.; Hompesch, M.; Kwon, S. Potent weight loss mechanism and improvement of NASH by the long-acting GLP-1/glucagon receptor dual agonist HM12525A. In Proceedings of the European Association for the Study of Diabetes, 51st Annual Meeting, Stockholm, Sweden, 14–18 September 2015. [Google Scholar]
- Robertson, D.; Hansen, L.; Ambery, P.; Esterline, R.L.; Jermutus, L.; Chang, Y.-T.; Petrone, M.; Johansson, E.; Johansson, L.; Sjöberg, F.B.; et al. 354-OR: Cotadutide (medi0382), a Dual Receptor Agonist with Glucagon-Like Peptide-1 and Glucagon Activity, Modulates Hepatic Glycogen and Fat Content. Diabetes 2020, 69, 354. [Google Scholar] [CrossRef]
- Tan, T.; Field, B.C.; McCullough, K.A.; Troke, R.C.; Chambers, E.S.; Salem, V.; Maffe, J.G.; Baynes, K.C.; De Silva, A.; Viardot, A.; et al. Coadministration of Glucagon-Like Peptide-1 During Glucagon Infusion in Humans Results in Increased Energy Expenditure and Amelioration of Hyperglycemia. Diabetes 2013, 62, 1131–1138. [Google Scholar] [CrossRef]
- Cegla, J.; Troke, R.C.; Jones, B.; Tharakan, G.; Kenkre, J.; McCullough, K.A.; Lim, C.T.; Parvizi, N.; Hussein, M.; Chambers, E.S.; et al. Coinfusion of Low-Dose GLP-1 and Glucagon in Man Results in a Reduction in Food Intake. Diabetes 2014, 63, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Bagger, J.I.; Holst, J.J.; Hartmann, B.; Andersen, B.; Knop, F.K.; Vilsbøll, T. Effect of Oxyntomodulin, Glucagon, GLP-1, and Combined Glucagon +GLP-1 Infusion on Food Intake, Appetite, and Resting Energy Expenditure. J. Clin. Endocrinol. Metab. 2015, 100, 4541–4552. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, B.; Larsen, O.; Gabe, M.B.N.; Christiansen, C.B.; Rosenkilde, M.M.; Drucker, D.J.; Holst, J.J. Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Rep. 2018, 25, 1127–1134.e2. [Google Scholar] [CrossRef]
- Moens, K.; Flamez, D.; Schravendijk, C.V.; Ling, Z.; Pipeleers, D.; Schuit, F. Dual Glucagon Recognition by Pancreatic beta-Cells via Glucagon and Glucagon-Like Peptide 1 Receptors. Diabetes 1998, 47, 66–72. [Google Scholar] [CrossRef]
- Panjwani, N.; Mulvihill, E.E.; Longuet, C.; Yusta, B.; Campbell, J.E.; Brown, T.J.; Streutker, C.; Holland, D.; Cao, X.; Baggio, L.L.; et al. GLP-1 Receptor Activation Indirectly Reduces Hepatic Lipid Accumulation but Does Not Attenuate Development of Atherosclerosis in Diabetic Male ApoE−/− Mice. Endocrinology 2013, 154, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Golabi, P.; De Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Halliday, D.; Matthews, D.E.; Welle, S.L. Hyperglucagonemia during insulin deficiency accelerates protein catabolism. Am. J. Physiol. 1987, 253, E208–E213. [Google Scholar] [CrossRef] [PubMed]
- Couet, C.; Fukagawa, N.K.; Matthews, D.E.; Bier, D.M.; Young, V.R. Plasma amino acid kinetics during acute states of glucagon deficiency and excess in healthy adults. Am. J. Physiol. Metab. 1990, 258, E78–E85. [Google Scholar] [CrossRef]
- Flakoll, P.; Borel, M.; Wentzel, L.; Williams, P.; Lacy, D.; Abumrad, N. The role of glucagon in the control of protein and amino acid metabolism in vivo. Metabolism 1994, 43, 1509–1516. [Google Scholar] [CrossRef]
- Kraft, G.; Coate, K.C.; Winnick, J.J.; Dardevet, D.; Donahue, E.P.; Cherrington, A.D.; Williams, P.E.; Moore, M.C. Glucagon’s effect on liver protein metabolism in vivo. Am. J. Physiol. Metab. 2017, 313, E263–E272. [Google Scholar] [CrossRef]
- Boden, G.; Rezvani, I.; Owen, O.E. Effects of glucagon on plasma amino acids. J. Clin. Investig. 1984, 73, 785–793. [Google Scholar] [CrossRef]
- Dean, E.D. A Primary Role for Alpha Cells as Amino Acid Sensors. Diabetes 2019. [Google Scholar] [CrossRef]
- Solloway, M.J.; Madjidi, A.; Gu, C.; Easthamanderson, J.; Clarke, H.J.; Kljavin, N.M.; Zavala-Solorio, J.; Kates, L.; Friedman, B.; Brauer, M.J.; et al. Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of α-Cell Mass. Cell Rep. 2015, 12, 495–510. [Google Scholar] [CrossRef]
- Holst, J.J.; Albrechtsen, N.J.W.; Pedersen, J.; Knop, F.K. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver–α-Cell Axis. Diabetes 2017, 66, 235–240. [Google Scholar] [CrossRef]
- Kim, J.; Okamoto, H.; Huang, Z.; Anguiano, G.; Chen, S.; Liu, Q.; Cavino, K.; Xin, Y.; Na, E.; Hamid, R.; et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab. 2017, 25, 1348–1361.e8. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.D.; Li, M.; Prasad, N.; Wisniewski, S.N.; Von Deylen, A.; Spaeth, J.; Maddison, L.; Botros, A.; Sedgeman, L.R.; Bozadjieva, N.; et al. Interrupted Glucagon Signaling Reveals Hepatic α Cell Axis and Role for L-Glutamine in α Cell Proliferation. Cell Metab. 2017, 25, 1362–1373.e5. [Google Scholar] [CrossRef] [PubMed]
- Galsgaard, K.D.; Winther-Sørensen, M.; Ørskov, C.; Kissow, H.; Poulsen, S.S.; Vilstrup, H.; Prehn, C.; Adamski, J.; Jepsen, S.L.; Hartmann, B.; et al. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am. J. Physiol. Metab. 2018, 314, E93–E103. [Google Scholar] [CrossRef] [PubMed]
- Albrechtsen, N.J.W.; Færch, K.; Jensen, T.M.; Witte, D.R.; Pedersen, J.; Mahendran, Y.; Jonsson, A.E.; Galsgaard, K.D.; Winther-Sørensen, M.; Torekov, S.S.; et al. Evidence of a liver–alpha cell axis in humans: Hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 2018, 61, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Master, R.W.; Rezvani, I.; Palmer, J.P.; Lobe, T.E.; Owen, O.E. Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. J. Clin. Investig. 1980, 65, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Qureshi, S.A.; Brady, E.J.; Muise, E.S.; Candelore, M.R.; Jiang, G.; Li, Z.; Wu, M.S.; Yang, X.; Dallas-Yang, Q.; et al. Anti-Diabetic Efficacy and Impact on Amino Acid Metabolism of GRA1, a Novel Small-Molecule Glucagon Receptor Antagonist. PLoS ONE 2012, 7, e49572. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.S.; Rygg, M.O.; Kristiansen, V.B.; Olsen, B.H.; Serizawa, R.R.; Holst, J.J.; Madsbad, S.; Gluud, L.L.; Bendtsen, F.; Albrechtsen, N.J.W. Nonalcoholic Fatty Liver Disease Impairs the Liver–Alpha Cell Axis Independent of Hepatic Inflammation and Fibrosis. Hepatol. Commun. 2020, 4, 1610–1623. [Google Scholar] [CrossRef]
- Winther-Sørensen, M.; Galsgaard, K.D.; Santos, A.; Trammell, S.A.; Sulek, K.; Kuhre, R.E.; Pedersen, J.; Andersen, D.B.; Hassing, A.S.; Dall, M.; et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol. Metab. 2020, 101080. [Google Scholar] [CrossRef]
- Gar, C.; Haschka, S.J.; Kern-Matschilles, S.; Rauch, B.; Sacco, V.; Prehn, C.; Adamski, J.; Seissler, J.; Albrechtsen, N.J.W.; Holst, J.J.; et al. The liver–alpha cell axis associates with liver fat and insulin resistance: A validation study in women with non-steatotic liver fat levels. Diabetologia 2020, 1–9. [Google Scholar] [CrossRef]
- Felig, P. Amino Acid Metabolism in Man. Annu. Rev. Biochem. 1975, 44, 933–955. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Junker, A.E.; Christensen, M.; Hædersdal, S.; Wibrand, F.; Lund, A.M.; Galsgaard, K.D.; Holst, J.J.; Knop, F.K.; Vilsbøll, T. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am. J. Physiol. Liver Physiol. 2018, 314, G91–G96. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, P.L.; Vilstrup, H.; Rigbolt, K.; Suppli, M.P.; Sorensen, M.; Heeboll, S.; Veidal, S.S.; Knop, F.K.; Thomsen, K.L. Non-alcoholic fatty liver disease alters expression of genes governing hepatic nitrogen conversion. Liver Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, P.L.; Sørensen, M.; Grønbæk, H.; Hamilton-Dutoit, S.; Vilstrup, H.; Thomsen, K.L. Non-alcoholic fatty liver disease causes dissociated changes in metabolic liver functions. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Albrechtsen, N.J.W.; Pedersen, J.; Galsgaard, K.D.; Winther-Sorensen, M.; Suppli, M.P.; Janah, L.; Gromada, J.; Vilstrup, H.; Knop, F.K.; Holst, J.J. The liver-alpha cell axis and type 2 diabetes. Endocr. Rev. 2019. [Google Scholar] [CrossRef]
- Suppli, M.P.; Lund, A.; Bagger, J.I.; Vilsbøll, T.; Knop, F.K. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Med Hypotheses 2016, 86, 100–103. [Google Scholar] [CrossRef]
- Suppli, M.P.; Bagger, J.I.; Lund, A.; Demant, M.; Van Hall, G.; Strandberg, C.; Kønig, M.J.; Rigbolt, K.; Langhoff, J.L.; Albrechtsen, N.J.W.; et al. Glucagon Resistance at the Level of Amino Acid Turnover and Ureagenesis in Obese Subjects with Hepatic Steatosis. Diabetes 2018, 67, 147. [Google Scholar] [CrossRef]
- Schutz, Y. Protein Turnover, Ureagenesis and Gluconeogenesis. Int. J. Vitam. Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Ramnanan, C.J.; Edgerton, D.S.; Kraft, G.; Cherrington, A.D. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 2011, 13, 118–125. [Google Scholar] [CrossRef]
- Charbonneau, A.; Couturier, K.; Gauthier, M.-S.; Lavoie, J.-M. Evidence of Hepatic Glucagon Resistance Associated with Hepatic Steatosis: Reversal Effect of Training. Int. J. Sports Med. 2005, 26, 432–441. [Google Scholar] [CrossRef]
- Charbonneau, A.; Melancon, A.; Lavoie, C.; Lavoie, J.-M. Alterations in hepatic glucagon receptor density and in Gsα and Giα2 protein content with diet-induced hepatic steatosis: Effects of acute exercise. Am. J. Physiol. Metab. 2005, 289, E8–E14. [Google Scholar] [CrossRef][Green Version]
- Charbonneau, A.; Unson, C.G.; Lavoie, J.-M. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: Potential interaction with acute exercise. J. Physiol. 2007, 579, 255–267. [Google Scholar] [CrossRef]
- Ashford, T.P.; Porter, K.R. Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 1962, 12, 198–202. [Google Scholar] [CrossRef]
- Arstila, A.U.; Trump, B.F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am. J. Pathol. 1968, 53, 687–733. [Google Scholar]
- Deter, R.L.; Baudhuin, P.; De Duve, C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol. 1967, 35, C11–C16. [Google Scholar] [CrossRef] [PubMed]
- Deter, R.L. Quantitative characterization of dense body, autophagic vacuole, and acid phosphatase-bearing particle populations during the early phases of glucagon-induced autophagy in rat liver. J. Cell Biol. 1971, 48, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Guder, W.; Hepp, K.D.; Wieland, O. The catabolic action of glucagon in rat liver. The influence of age, nutritional state and adrenal function on the effect of glucagon on lysosomal N-acetyl-beta, D-glucosaminidase. Biochim. Biophys. Acta 1970, 222, 593–605. [Google Scholar] [CrossRef]
- Becker, F.F.; Cornwall, C.C. Phlorizin induced autophagocytosis during hepatocytic glycogenolysis. Exp. Mol. Pathol. 1971, 14, 103–109. [Google Scholar] [CrossRef]
- Amherdt, M.; Harris, V.; Renold, A.E.; Orci, L.; Unger, R.H. Hepatic Autography in Uncontrolled Experimental Diabetes and Its Relationships to Insulin and Glucagon. J. Clin. Investig. 1974, 54, 188–193. [Google Scholar] [CrossRef]
- Ruan, H.-B.; Ma, Y.; Torres, S.; Zhang, B.; Feriod, C.; Heck, R.M.; Qian, K.; Fu, M.; Li, X.; Nathanson, M.H.; et al. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017, 31, 1655–1665. [Google Scholar] [CrossRef]
- Ezaki, J.; Matsumoto, N.; Takeda-Ezaki, M.; Komatsu, M.; Takahashi, K.; Hiraoka, Y.; Taka, H.; Fujimura, T.; Takehana, K.; Yoshida, M.; et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy 2011, 7, 727–736. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.P.; Qian, T.; Grissom, S.F.; Lemasters, J.J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.K.; Zhang, L.; Chan, M.T.V. Autophagy, NAFLD and NAFLD-Related HCC. Adv. Exp. Med. Biol. 2018, 1061, 127–138. [Google Scholar] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Lavallard, V.J.; Gual, P. Autophagy and Non-Alcoholic Fatty Liver Disease. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, W.; Zhou, Y.; Li, F.; Wei, H.; Peng, J. Recent Advances in Understanding Amino Acid Sensing Mechanisms that Regulate mTORC1. Int. J. Mol. Sci. 2016, 17, 1636. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.-I.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 Association with the ULK1–Atg13–FIP200 Complex Required for Autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef]
| Metabolic Process | Pathology | |
|---|---|---|
| Amino acid/protein metabolism | Amino acid transport Amino acid catabolism Ureagenesis | Hyperaminoacidemia Hyperglucagonemia Hyperammonemia |
| Autophagy | Lipophagy Mitophagy | Increased hepatic fat Increased oxidative stress |
| Lipid metabolism | β-oxidation Lipolysis | Increased hepatic fat Dyslipidemia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galsgaard, K.D. The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2020, 9, 4049. https://doi.org/10.3390/jcm9124049
Galsgaard KD. The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine. 2020; 9(12):4049. https://doi.org/10.3390/jcm9124049
Chicago/Turabian StyleGalsgaard, Katrine D. 2020. "The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease" Journal of Clinical Medicine 9, no. 12: 4049. https://doi.org/10.3390/jcm9124049
APA StyleGalsgaard, K. D. (2020). The Vicious Circle of Hepatic Glucagon Resistance in Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine, 9(12), 4049. https://doi.org/10.3390/jcm9124049




