AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas
Abstract
:1. Introduction
1.1. Endometrial Cancer
1.2. Ovarian Cancer
1.3. Biomarkers for Endometrial and Ovarian Cancers
1.4. Aldo-Keto Reductase 1C3
2. Materials and Methods
2.1. Study Groups
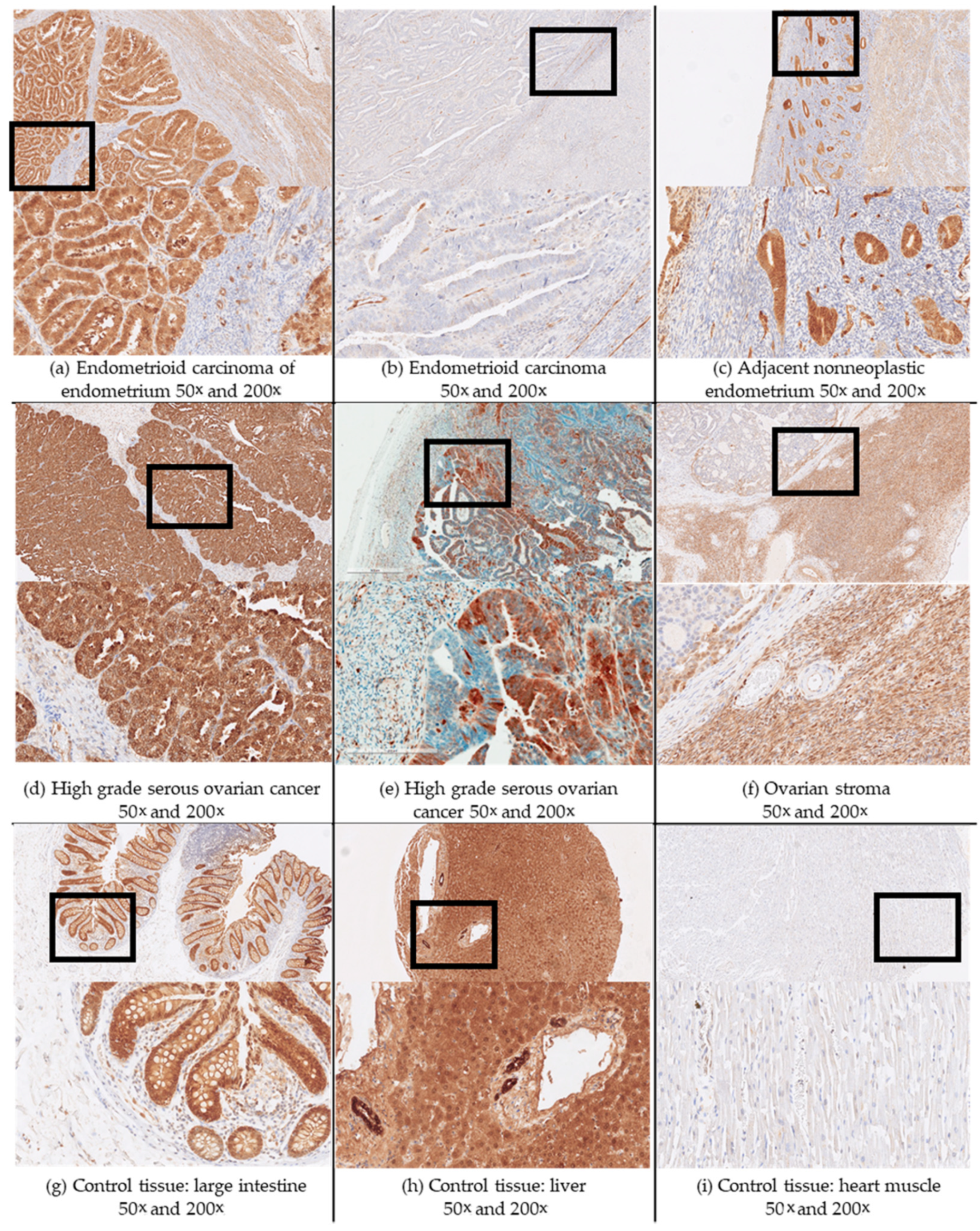
2.2. Immunohistochemistry
2.3. Statistics
2.4. Ethical Issues
3. Results
3.1. Demographic and Histopathological Characteristics of Patients with Endometrial and Ovarian Cancers
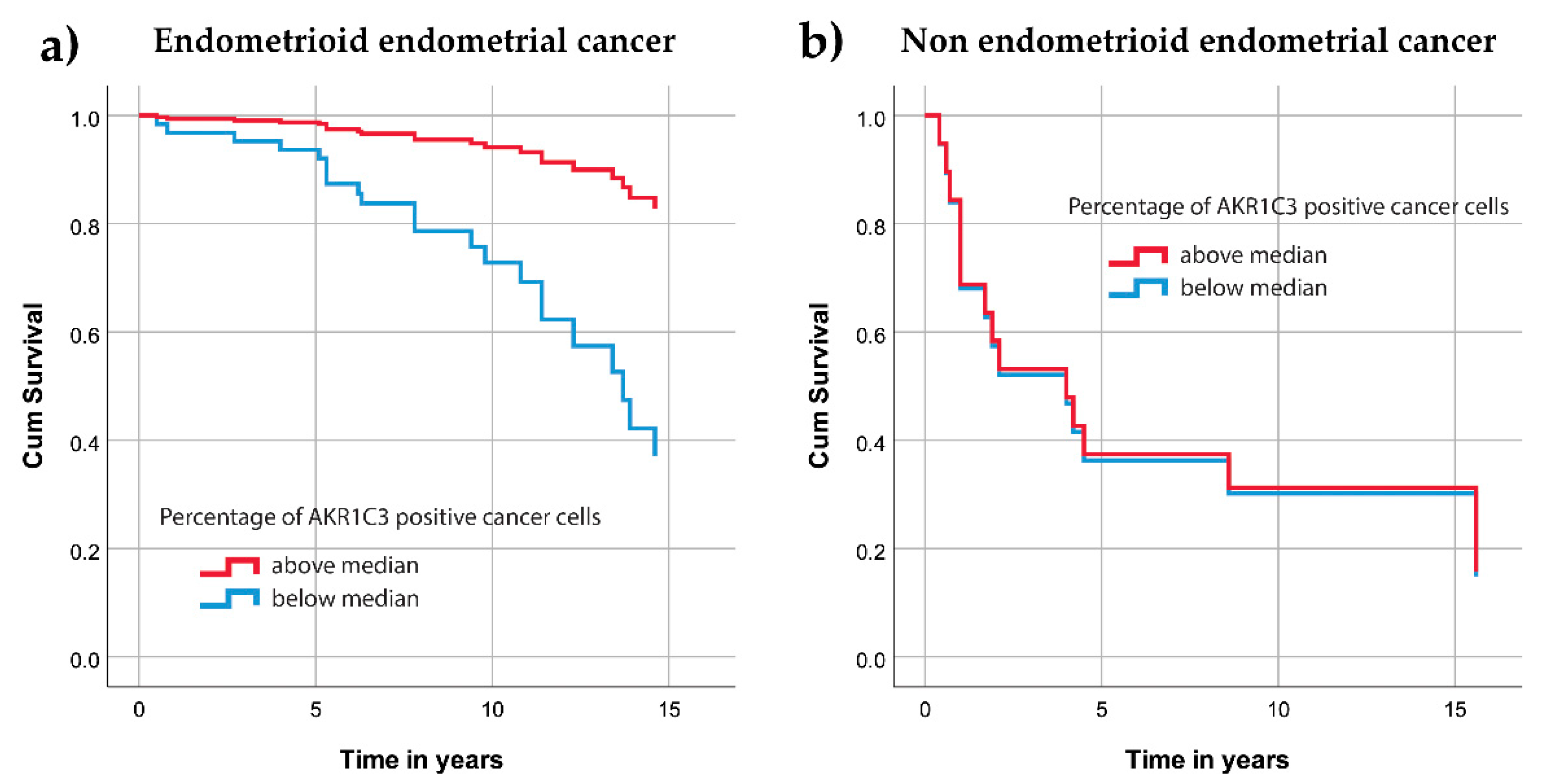
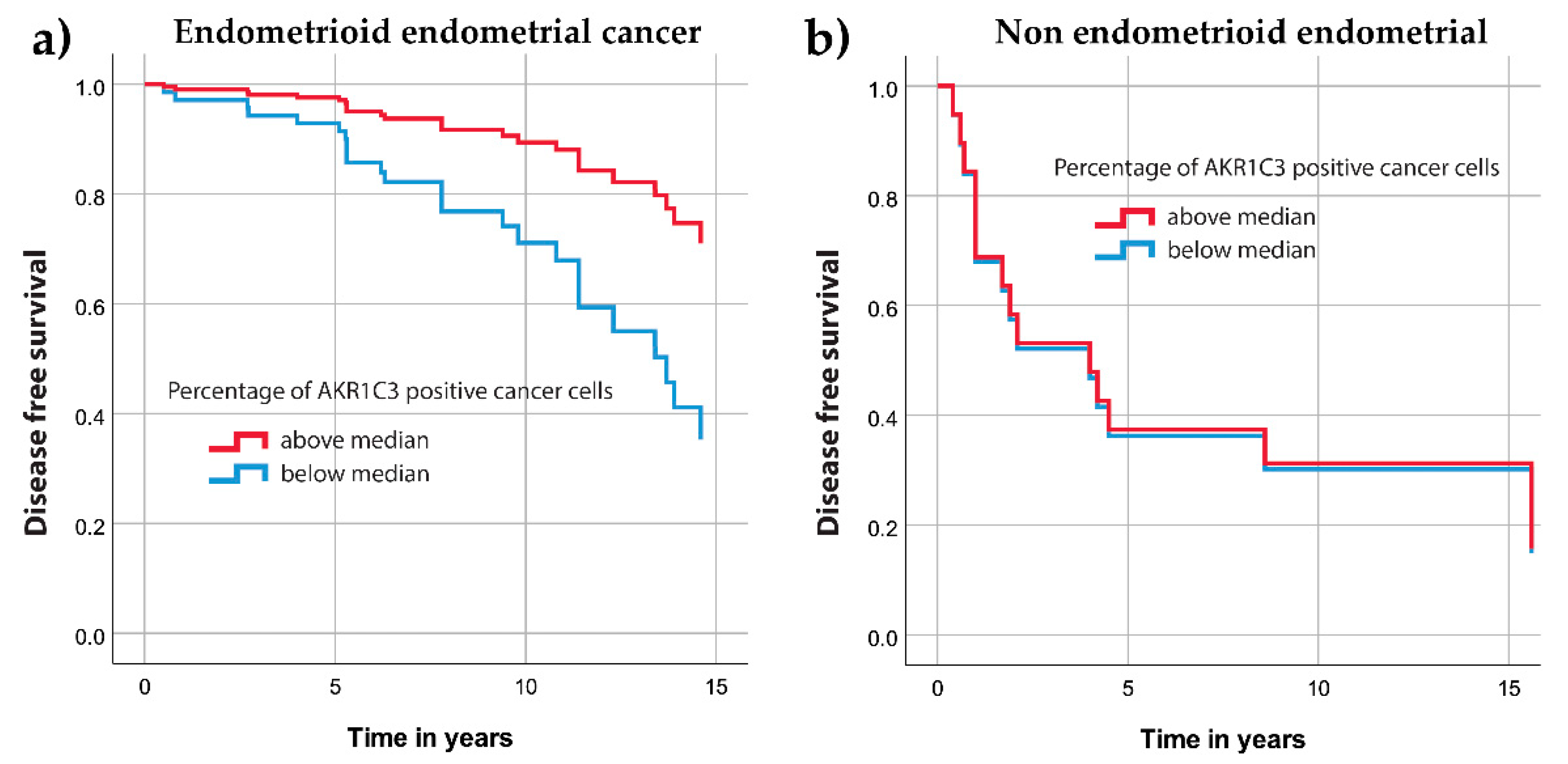
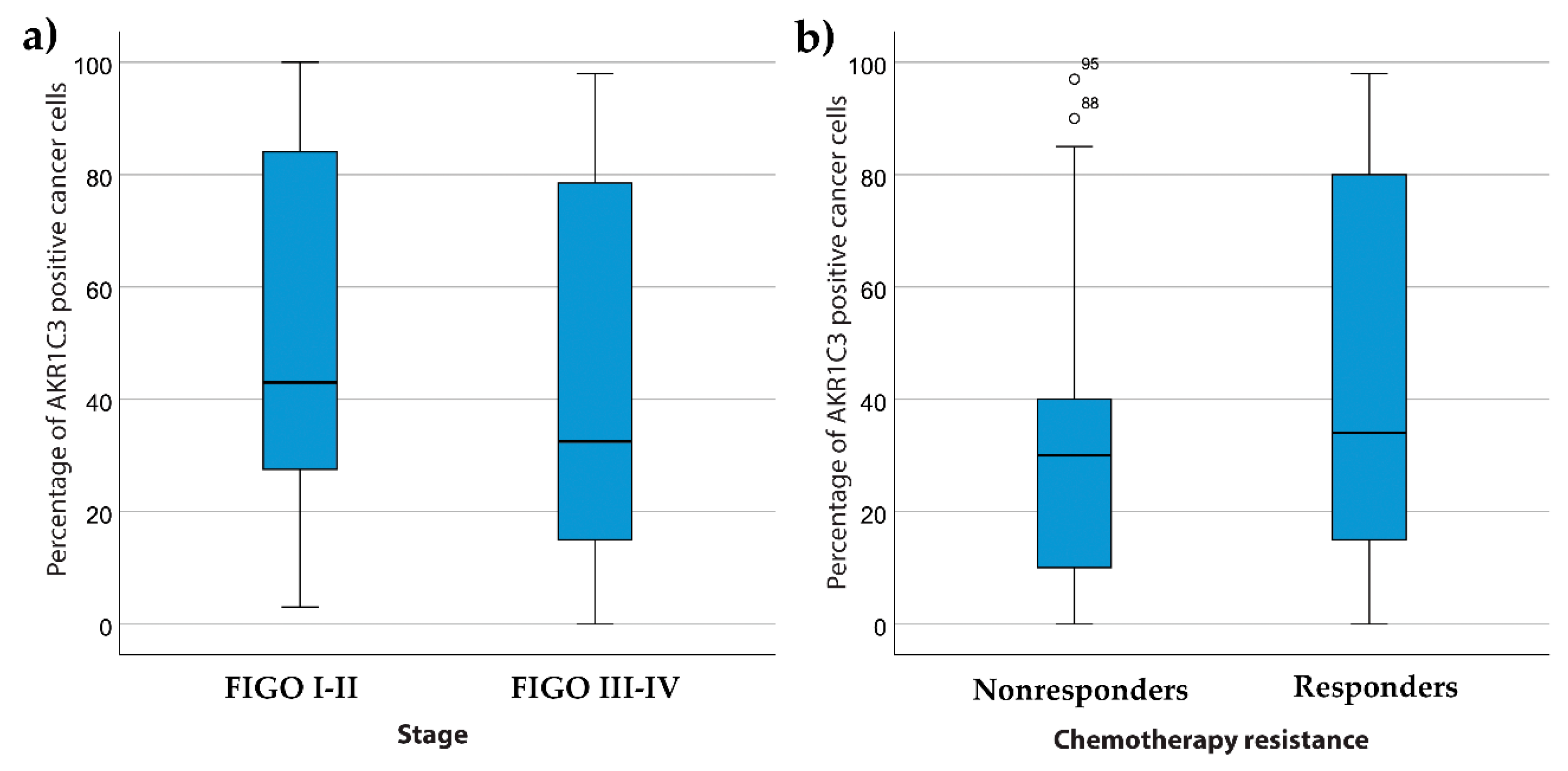
3.2. AKR1C3 Expression Levels in Endometrial Cancer and Their Correlation with Survival and Other Clinical Data
3.3. AKR1C3 Levels in Ovarian Cancer and Their Correlations with Survival and Other Clinical Data
4. Discussion
4.1. AKR1C3 in Endometrial Cancer
4.2. AKR1C3 in Ovarian Cancer
4.3. Importance of AKR1C3 in Endometrial and Ovarian Cancers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Persson, I. Estrogens in the causation of breast, endometrial and ovarian cancers—evidence and hypotheses from epidemiological findings. J. Steroid Biochem. Mol. Biol. 2000, 74, 357–364. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- OI, L. Slovenian Cancer Registry, Epidemiology and Cancer Registry, Cancer in Slovenia 2017; Institute of Oncology Ljubljana: Ljubljana, Slovenia, 2020. [Google Scholar]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II endometrial cancers: Have they different risk factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, S.; Coward, J.I.; Bast, R.C.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Rižner, T.L. Discovery of biomarkers for endometrial cancer: Current status and prospects. Expert Rev. Mol. Diagn. 2016, 16, 1315–1336. [Google Scholar] [CrossRef]
- Muinao, T.; Deka Boruah, H.P.; Pal, M. Diagnostic and Prognostic Biomarkers in ovarian cancer and the potential roles of cancer stem cells—An updated review. Exp. Cell Res. 2018, 362, 1–10. [Google Scholar] [CrossRef]
- Sun, S.Q.; Gu, X.; Gao, X.S.; Li, Y.; Yu, H.; Xiong, W.; Wang, W.; Teng, Y.; Zhou, D. Overexpression of AKR1C3 significantly enhances human prostate cancer cells resistance to radiation. Oncotarget 2016, 7, 48050–48058. [Google Scholar] [CrossRef] [Green Version]
- Penning, T.M.; Byrns, M.C. Steroid hormone transforming aldo-keto reductases and cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Penning, T.M.; Wangtrakuldee, P.; Auchus, R.J. Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes. Endocr. Rev. 2019, 40, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Byrns, M.C.; Duan, L.; Lee, S.H.; Blair, I.A.; Penning, T.M. Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J. Steroid Biochem. Mol. Biol. 2010, 118, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penning, T.M. AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase): Roles in malignancy and endocrine disorders. Mol. Cell Endocrinol. 2019, 489, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L.; Penning, T.M. Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 2014, 79, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Gu, Y.; Hui, K.; Huang, J.; Xu, S.; Wu, S.; Li, L.; Fan, J.; Wang, X.; Hsieh, J.T.; et al. AKR1C3, a crucial androgenic enzyme in prostate cancer, promotes epithelial-mesenchymal transition and metastasis through activating ERK signaling. Urol. Oncol. 2018, 36, 472.e11–472.e20. [Google Scholar] [CrossRef]
- Chen, J.; Adikari, M.; Pallai, R.; Parekh, H.K.; Simpkins, H. Dihydrodiol dehydrogenases regulate the generation of reactive oxygen species and the development of cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother. Pharm. 2008, 61, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.B.; Adikari, M.; Parekh, H.K.; Simpkins, H. Ubiquitous induction of resistance to platinum drugs in human ovarian, cervical, germ-cell and lung carcinoma tumor cells overexpressing isoforms 1 and 2 of dihydrodiol dehydrogenase. Cancer Chemother. Pharm. 2004, 54, 301–307. [Google Scholar] [CrossRef]
- Penning, T.M. Aldo-Keto Reductase Regulation by the Nrf2 System: Implications for Stress Response, Chemotherapy Drug Resistance, and Carcinogenesis. Chem. Res. Toxicol. 2017, 30, 162–176. [Google Scholar] [CrossRef]
- Hofman, J.; Malcekova, B.; Skarka, A.; Novotna, E.; Wsol, V. Anthracycline resistance mediated by reductive metabolism in cancer cells: The role of aldo-keto reductase 1C3. Toxicol. Appl. Pharm. 2014, 278, 238–248. [Google Scholar] [CrossRef]
- Bortolozzi, R.; Bresolin, S.; Rampazzo, E.; Paganin, M.; Maule, F.; Mariotto, E.; Boso, D.; Minuzzo, S.; Agnusdei, V.; Viola, G.; et al. AKR1C enzymes sustain therapy resistance in paediatric T-ALL. Br. J. Cancer 2018, 118, 985–994. [Google Scholar] [CrossRef]
- Bukum, N.; Novotna, E.; Morell, A.; Hofman, J.; Wsol, V. Buparlisib is a novel inhibitor of daunorubicin reduction mediated by aldo-keto reductase 1C3. Chem. Biol. Interact. 2019, 302, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K. Treatment for recurrent ovarian cancer-at first relapse. J. Oncol. 2010, 2010, 497429. [Google Scholar] [CrossRef] [PubMed]
- Hevir-Kene, N.; Rižner, T.L. The endometrial cancer cell lines Ishikawa and HEC-1A, and the control cell line HIEEC, differ in expression of estrogen biosynthetic and metabolic genes, and in androstenedione and estrone-sulfate metabolism. Chem. Biol. Interact. 2015, 234, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Catalano, R.D.; Wilson, M.R.; Boddy, S.C.; Jabbour, H.N. Comprehensive expression analysis of prostanoid enzymes and receptors in the human endometrium across the menstrual cycle. Mol. Hum. Reprod. 2011, 17, 182–192. [Google Scholar] [CrossRef]
- Pelletier, G.; Luu-The, V.; Têtu, B.; Labrie, F. Immunocytochemical localization of type 5 17beta-hydroxysteroid dehydrogenase in human reproductive tissues. J. Histochem. Cytochem. 1999, 47, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Utsunomiya, H.; Suzuki, T.; Saitou, S.; Akahira, J.; Okamura, K.; Yaegashi, N.; Sasano, H. 17Beta-hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol. Cell Endocrinol. 2006, 248, 136–140. [Google Scholar] [CrossRef]
- Sinreih, M.; Hevir, N.; Rižner, T.L. Altered expression of genes involved in progesterone biosynthesis, metabolism and action in endometrial cancer. Chem. Biol. Interact. 2013, 202, 210–217. [Google Scholar] [CrossRef]
- Rizner, T.L.; Smuc, T.; Rupreht, R.; Sinkovec, J.; Penning, T.M. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol. Cell Endocrinol. 2006, 248, 126–135. [Google Scholar] [CrossRef]
- Zakharov, V.; Lin, H.K.; Azzarello, J.; McMeekin, S.; Moore, K.N.; Penning, T.M.; Fung, K.M. Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int. J. Clin. Exp. Pathol. 2010, 3, 608–617. [Google Scholar]
- Chura, J.C.; Ryu, H.S.; Simard, M.; Poirier, D.; Tremblay, Y.; Brooker, D.C.; Blomquist, C.H.; Argenta, P.A. Steroid-converting enzymes in human ovarian carcinomas. Mol. Cell Endocrinol. 2009, 301, 51–58. [Google Scholar] [CrossRef]
- Ren, X.; Wu, X.; Hillier, S.G.; Fegan, K.S.; Critchley, H.O.; Mason, J.I.; Sarvi, S.; Harlow, C.R. Local estrogen metabolism in epithelial ovarian cancer suggests novel targets for therapy. J. Steroid Biochem. Mol. Biol. 2015, 150, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, S.R.; Narendrula, R.; Guo, B.; Parissenti, A.M.; McCallum, K.L.; Cull, S.; Lannér, C. Distinct genetic alterations occur in ovarian tumor cells selected for combined resistance to carboplatin and docetaxel. J. Ovarian Res. 2012, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Bell, C.C.; Gilan, O. Principles and mechanisms of non-genetic resistance in cancer. Br. J. Cancer 2019. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, H.; Huang, C.; Lei, Y. Cancer drug resistance: Redox resetting renders a way. Oncotarget 2016, 7, 42740–42761. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Hackenberg, R.; Schulz, K.D. Androgen receptor mediated growth control of breast cancer and endometrial cancer modulated by antiandrogen- and androgen-like steroids. J. Steroid Biochem. Mol. Biol. 1996, 56, 113–117. [Google Scholar] [CrossRef]
- Gibson, D.A.; Simitsidellis, I.; Collins, F.; Saunders, P.T. Evidence of androgen action in endometrial and ovarian cancers. Endocr. Relat. Cancer 2014, 21, T203–T218. [Google Scholar] [CrossRef]
- Mizushima, T.; Miyamoto, H. The Role of Androgen Receptor Signaling in Ovarian Cancer. Cells 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, Z.; Abdulfatah, E.; Pardeshi, V.; Hassan, O.; Schultz, D.; Morris, R.; Cote, M.L.; Elshaikh, M.A.; Bandyopadhyay, S.; Ali-Fehmi, R. The Impact of Androgen Receptor Expression on Endometrial Carcinoma Recurrence and Survival. Int. J. Gynecol. Pathol. 2017, 36, 405–411. [Google Scholar] [CrossRef]
- Elattar, A.; Warburton, K.G.; Mukhopadhyay, A.; Freer, R.M.; Shaheen, F.; Cross, P.; Plummer, E.R.; Robson, C.N.; Edmondson, R.J. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol. Oncol. 2012, 124, 142–147. [Google Scholar] [CrossRef]




| Characteristic | Detail | Datum |
|---|---|---|
| Age (y) | Mean ± SD | 63.5 ± 11.0 |
| Weight (kg) | Mean ± SD | 82.1 ± 18.2 |
| Height (cm) | Mean ± SD | 161.9 ± 5.6 |
| Body mass index (kg/m2) | Mean ± SD | 31.4 ± 7.2 |
| Menopausal status (N = 118)a (n (%)) | Postmenopausal | 102 (86.4) |
| Histological type (N = 123) (n (%)) | Endometrioid | 104 (84.6) |
| Serous | 12 (9.8) | |
| Dedifferentiated | 3 (2.4) | |
| Carcinosarcoma | 2 (1.6) | |
| Clear cell | 1 (0.8) | |
| Mixed carcinoma | 1 (0.8) | |
| Histological grade (N = 104) (n (%)) | G1 | 67 (64.4) |
| G2 | 25 (24.0) | |
| G3 | 12 (11.5) | |
| Myometrial invasion (n (%)) | <50% | 90 (73.1) |
| ≥50% | 33 (26.8) | |
| Lymphovascular invasion (n (%)) | 34 (27.6) | |
| FIGO stage (N = 117) a (n (%)) | I–II | 104 (84.6) |
| III–IV | 13 (10.6) | |
| Follow-up (y) | Range | 0.4–17.6 |
| Median | 7.6 | |
| 5-year survival (n (%)) | 107 (87.0) |
| Characteristic | Detail | Datum |
|---|---|---|
| Age (y) | Mean ± SD | 61.5 ± 11.4 |
| Ascites (n (%)) | 54 (54.5) | |
| Chemotherapy with reported follow-up (N = 71) a (n (%)) | Responders (at least 6 months DFS) | 53 (74.6) |
| Non-responders (6 months DFS was not achieved) | 18 (25.4) | |
| FIGO stage (N = 99) | I–II | 19 |
| III–IV | 80 | |
| Follow-up (y) | Range | 0.3–11.3 |
| Median | 3.1 | |
| 5-year survival rate a (n (%)) | 29 (29.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojnik, M.; Kenda Šuster, N.; Smrkolj, Š.; Frković Grazio, S.; Verdenik, I.; Rižner, T.L. AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas. J. Clin. Med. 2020, 9, 4105. https://doi.org/10.3390/jcm9124105
Hojnik M, Kenda Šuster N, Smrkolj Š, Frković Grazio S, Verdenik I, Rižner TL. AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas. Journal of Clinical Medicine. 2020; 9(12):4105. https://doi.org/10.3390/jcm9124105
Chicago/Turabian StyleHojnik, Marko, Nataša Kenda Šuster, Špela Smrkolj, Snježana Frković Grazio, Ivan Verdenik, and Tea Lanišnik Rižner. 2020. "AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas" Journal of Clinical Medicine 9, no. 12: 4105. https://doi.org/10.3390/jcm9124105
APA StyleHojnik, M., Kenda Šuster, N., Smrkolj, Š., Frković Grazio, S., Verdenik, I., & Rižner, T. L. (2020). AKR1C3 Is Associated with Better Survival of Patients with Endometrial Carcinomas. Journal of Clinical Medicine, 9(12), 4105. https://doi.org/10.3390/jcm9124105






