The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children
Abstract
1. Introduction
2. Materials and Methods
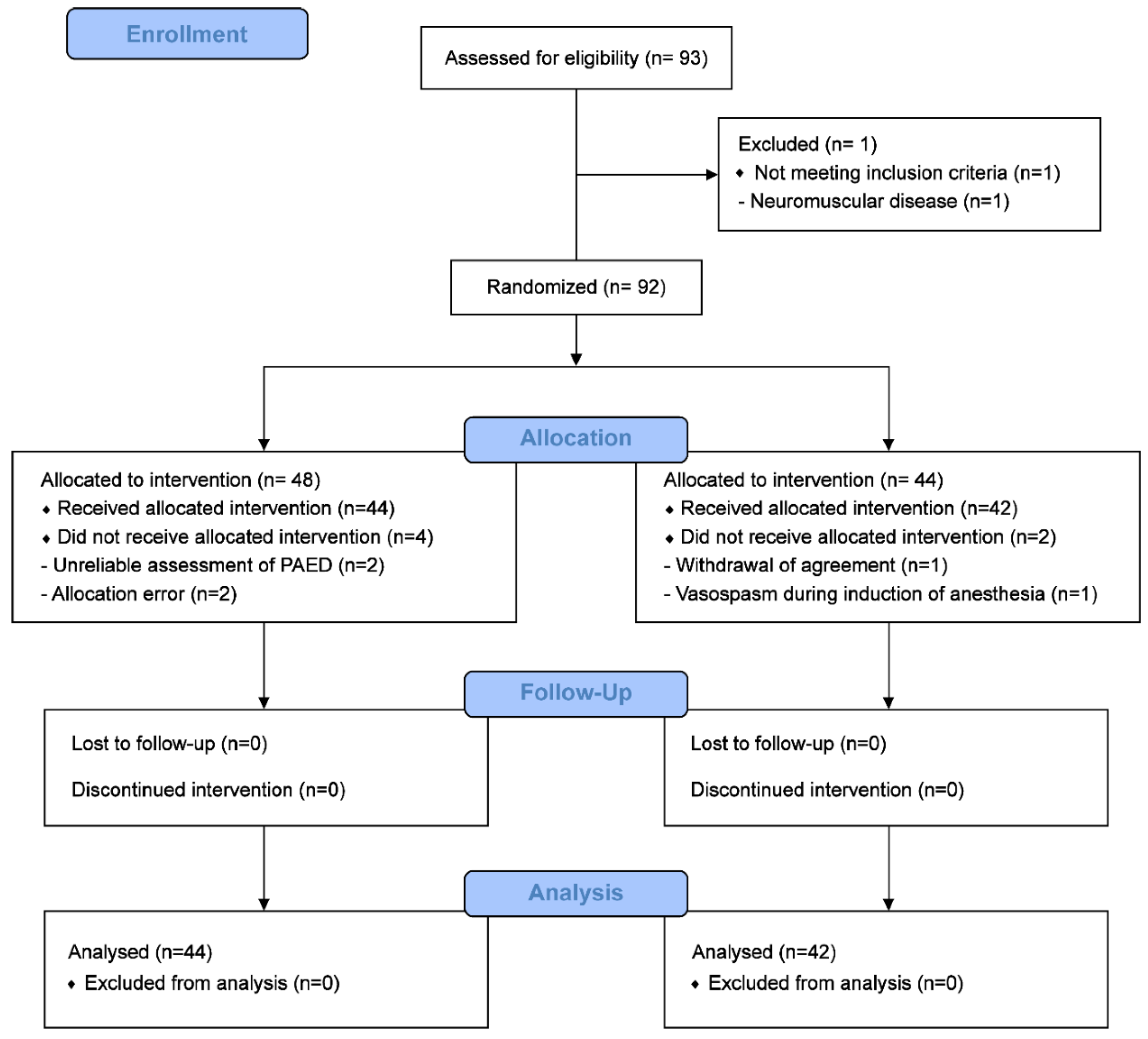
2.1. Study Design, Participants and Recruitment
2.2. Interventions and Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, J.W.; Murray, D.J.; McAllister, J.D.; Hirshberg, G.E. Emergence behaviour in children: Defining the incidence of excitement and agitation following anaesthesia. Paediatr. Anaesth. 2002, 12, 442–447. [Google Scholar] [CrossRef]
- Kanaya, A. Emergence agitation in children: Risk factors, prevention, and treatment. J. Anesth. 2016, 30, 261–267. [Google Scholar] [CrossRef]
- Abdulatif, M.; Ahmed, A.; Mukhtar, A.; Badawy, S. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia 2013, 68, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Costi, D.; Cyna, A.M.; Ahmed, S.; Stephens, K.; Strickland, P.; Ellwood, J.; Larsson, J.N.; Chooi, C.; Burgoyne, L.L.; Middleton, P. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Uezono, S.; Goto, T.; Terui, K.; Ichinose, F.; Ishguro, Y.; Nakata, Y.; Morita, S. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth. Analg. 2000, 91, 563–566. [Google Scholar] [CrossRef]
- Dahmani, S.; Stany, I.; Brasher, C.; Lejeune, C.; Bruneau, B.; Wood, C.; Nivoche, Y.; Constant, I.; Murat, I. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: A meta-analysis of published studies. Br. J. Anaesth. 2010, 104, 216–223. [Google Scholar] [CrossRef]
- Abu-Shahwan, I.; Chowdary, K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr. Anaesth. 2007, 17, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Ueda, W.; Mamiya, K.; Takimoto, E.; Manabe, M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997, 87, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Cravero, J.P.; Beach, M.; Thyr, B.; Whalen, K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth. Analg. 2003, 97, 364–367. [Google Scholar] [CrossRef]
- Davis, P.J.; Cohen, I.T.; McGowan, F.X., Jr.; Latta, K. Recovery characteristics of desflurane versus halothane for maintenance of anesthesia in pediatric ambulatory patients. Anesthesiology 1994, 80, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Sikich, N.; Lerman, J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004, 100, 1138–1145. [Google Scholar] [CrossRef]
- Aouad, M.T.; Yazbeck-Karam, V.G.; Nasr, V.G.; El-Khatib, M.F.; Kanazi, G.E.; Bleik, J.H. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology 2007, 107, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.L.; Ng, A.S. Evaluation of emergence delirium in Asian children using the Pediatric Anesthesia Emergence Delirium Scale. Paediatr. Anaesth. 2009, 19, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.Y.; Lee, J.H.; Kang, Y.R.; Koo, B.N. Low-dose dexmedetomidine reduces emergence agitation after desflurane anaesthesia in children undergoing strabismus surgery. Yonsei Med. J. 2014, 55, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Voepel-Lewis, T.; Malviya, S.; Tait, A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth. Analg. 2003, 96, 1625–1630. [Google Scholar] [CrossRef]
- Veyckemans, F. Excitation phenomena during sevoflurane anaesthesia in children. Curr. Opin. Anaesthesiol. 2001, 14, 339–343. [Google Scholar] [CrossRef]
- Mizrak, A.; Erbagci, I.; Arici, T.; Ozcan, I.; Ganidagli, S.; Tatar, G.; Oner, U. Ketamine versus propofol for strabismus surgery in children. Clin. Ophthalmol. 2010, 4, 673–679. [Google Scholar] [CrossRef]
- Shin, D.H.; Woo, K.I.; Kim, Y.D. Relationship between lower eyelid epiblepharon and epicanthus in Korean children. PLoS ONE 2017, 12, e0187690. [Google Scholar] [CrossRef]
- Woo, K.I.; Yi, K.; Kim, Y.D. Surgical correction for lower lid epiblepharon in Asians. Br. J. Ophthalmol. 2000, 84, 1407–1410. [Google Scholar] [CrossRef]
- Oh, A.Y.; Seo, K.S.; Kim, S.D.; Kim, C.S.; Kim, H.S. Delayed emergence process does not result in a lower incidence of emergence agitation after sevoflurane anesthesia in children. Acta Anaesthesiol. Scand. 2005, 49, 297–299. [Google Scholar] [CrossRef]
- Cohen, I.T.; Finkel, J.C.; Hannallah, R.S.; Hummer, K.A.; Patel, K.M. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: A comparison with propofol. Paediatr. Anaesth. 2003, 13, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jacob, Z.; Li, H.; Makaryus, R.; Zhang, S.; Reinsel, R.; Lee, H.; Feng, T.; Rothman, D.L.; Benveniste, H. Metabolomic profiling of children’s brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiology 2012, 117, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Bariskaner, H.; Ustun, M.E.; Ak, A.; Yosunkaya, A.; Ulusoy, H.B.; Gurbilek, M. Effects of magnesium sulfate on tissue lactate and malondialdehyde levels after cerebral ischemia. Pharmacology 2003, 68, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Chung, S.Y.; Lin, M.C.; Cheng, F.C. Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002, 71, 803–811. [Google Scholar] [CrossRef]
- Pender, J.W. Dissociative anesthesia. Calif. Med. 1972, 117, 46–47. [Google Scholar]
- White, P.F.; Way, W.L.; Trevor, A.J. Ketamine--its pharmacology and therapeutic uses. Anesthesiology 1982, 56, 119–136. [Google Scholar] [CrossRef]
- Gardoni, F.; Di Luca, M. New targets for pharmacological intervention in the glutamatergic synapse. Eur. J. Pharmacol. 2006, 545, 2–10. [Google Scholar] [CrossRef]
- Raffa, R.B.; Ortegón, M.E.; Robisch, D.M.; Martin, G.E. In vivo demonstration of the enhancement of MK-801 by L-glutamate. Life Sci. 1989, 44, 1593–1599. [Google Scholar] [CrossRef]
- Schmidt, W.J.; Bury, D. Behavioural effects of N-methyl-D-aspartate in the anterodorsal striatum of the rat. Life Sci. 1988, 43, 545–549. [Google Scholar] [CrossRef]
- Orser, B.A.; Bertlik, M.; Wang, L.Y.; MacDonald, J.F. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br. J. Pharm. 1995, 116, 1761–1768. [Google Scholar] [CrossRef]
- Coutinho, E.M. Calcium, magnesium, and local anesthesia. J. Gen. Physiol. 1966, 49, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, S.J.; Lucas, D.R. Physiological and Pharmacological Studies of Magnesium Salts: V. the Influence of Nephrectomy Upon Their Toxicity. J. Exp. Med. 1907, 9, 298–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peck, C.H.; Meltzer, S.J. Anesthesia in Human Beings by Intravenous Injection of Magnesium Sulphate. J. Am. Med. Assoc. 1916, LXVII, 1131–1133. [Google Scholar] [CrossRef][Green Version]
- Bilotta, F.; Gelb, A.W.; Stazi, E.; Titi, L.; Paoloni, F.P.; Rosa, G. Pharmacological perioperative brain neuroprotection: A qualitative review of randomized clinical trials. Br. J. Anaesth. 2013, 110, i113–i120. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef]
- Xie, M.; Li, X.-k.; Peng, Y. Magnesium sulfate for postoperative complications in children undergoing tonsillectomies: A systematic review and meta-analysis. J. Evid. Based Med. 2017, 10, 16–25. [Google Scholar] [CrossRef]
- Bortone, L.; Bertolizio, G.; Engelhardt, T.; Frawley, G.; Somaini, M.; Ingelmo, P.M. The effect of fentanyl and clonidine on early postoperative negative behavior in children: A double-blind placebo controlled trial. Paediatr. Anaesth. 2014, 24, 614–619. [Google Scholar] [CrossRef]
- Honarmand, A.; Safavi, M.; Badiei, S.; Daftari-Fard, N. Different doses of intravenous Magnesium sulfate on cardiovascular changes following the laryngoscopy and tracheal intubation: A double-blind randomized controlled trial. J. Res. Pharm. Pract. 2015, 4, 79–84. [Google Scholar] [CrossRef]
- Taheri, A.; Haryalchi, K.; Mansour Ghanaie, M.; Habibi Arejan, N. Effect of low-dose (single-dose) magnesium sulfate on postoperative analgesia in hysterectomy patients receiving balanced general anesthesia. Anesth. Res. Pract. 2015, 2015, 306145. [Google Scholar] [CrossRef]
- Ryu, J.H.; Sohn, I.S.; Do, S.H. Controlled hypotension for middle ear surgery: A comparison between remifentanil and magnesium sulphate. Br. J. Anaesth. 2009, 103, 490–495. [Google Scholar] [CrossRef]
- Ecoffey, B. Recovery and outcome after minor pediatric surgery. Minerva Anestesiol. 2002, 68, 392–395. [Google Scholar] [PubMed]
- Pereira, L.; Figueiredo-Braga, M.; Carvalho, I.P. Preoperative anxiety in ambulatory surgery: The impact of an empathic patient-centered approach on psychological and clinical outcomes. Patient Educ. Couns. 2016, 99, 733–738. [Google Scholar] [CrossRef] [PubMed]
| Item |
|---|
| A. The child makes eye contact with caregiver |
| B. The child’s actions are purposeful |
| C. The child is aware of his/her surroundings |
| D. The child is restless |
| E. The child is inconsolable |
| Control (n = 44) | Magnesium (n = 42) | p Value | |
|---|---|---|---|
| Male, n (%) | 25 (56.8) | 16 (38.1) | 0.082 |
| Age (year) | 5 (4–6) | 5 (4–6) | 0.548 |
| Weight (kg) | 19.1 (16.8–23.7) | 21.3 (18.6–26.0) | 0.136 |
| Preop. Mg (mmol/L) | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.321 |
| Postop. Mg (mmol/L) | 0.5 (0.5–0.5) | 0.8 (0.7–0.9) | <0.001 * |
| Intraoperative MBP (mmHg) | 60 ± 9 | 60 ± 9 | 0.953 |
| Intraoperative MHR (bpm) | 101 ± 14 | 98 ± 14 | 0.396 |
| ASA status, n (%) | 1.000 | ||
| ASA I | 41 (93.2) | 39 (92.9) | |
| ASA II | 3 (6.8) | 3 (7.1) | |
| Anaesthesia time (min) | 40 (30–45) | 35 (30–46) | 0.456 |
| Operation time (min) | 20 (20–30) | 20 (15–31) | 0.471 |
| Mean sevo. (vol%) | 3.0 (3.0–3.0) | 3.0 (3.0–3.0) | 0.816 |
| Control (n = 44) | Magnesium (n = 42) | p Value | |
|---|---|---|---|
| Incidence of EA, n (%) | |||
| PAED 0 | 34 (77.3) | 24 (57.1) | 0.046 * |
| PAED 15 | 9 (20.5) | 4 (9.5) | 0.526 |
| PAED 30 | 0 (0) | 0 (0) | 1.0 |
| PAED scale | |||
| PAED 0 | 15.0 (10.0–17.0) | 11.5 (7.0–15.3) | 0.019 * |
| PAED 15 | 7.3 ± 3.9 | 6.8 ± 2.6 | 0.559 |
| PAED 30 | 6.8 ± 1.6 | 7.3 ± 1.0 | 0.592 |
| Total rescue dose of fentanyl (μg) | 12.0 (6.5–18.0) | 9.8 (0–15.5) | 0.141 |
| PACU time (min) | 31 (28–35) | 30 (27–36) | 0.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Kim, B.-Y.; Park, J.-H.; Kim, S.-Y.; Park, H.-Y.; Do, S.-H. The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children. J. Clin. Med. 2020, 9, 4126. https://doi.org/10.3390/jcm9124126
Lee Y-J, Kim B-Y, Park J-H, Kim S-Y, Park H-Y, Do S-H. The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children. Journal of Clinical Medicine. 2020; 9(12):4126. https://doi.org/10.3390/jcm9124126
Chicago/Turabian StyleLee, Yea-Ji, Bo-Young Kim, Jae-Hee Park, Sae-Yeon Kim, Hee-Yeon Park, and Sang-Hwan Do. 2020. "The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children" Journal of Clinical Medicine 9, no. 12: 4126. https://doi.org/10.3390/jcm9124126
APA StyleLee, Y.-J., Kim, B.-Y., Park, J.-H., Kim, S.-Y., Park, H.-Y., & Do, S.-H. (2020). The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children. Journal of Clinical Medicine, 9(12), 4126. https://doi.org/10.3390/jcm9124126




