Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Outcome Variables
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results and Trial Flow
3.2. Characteristics of Included Studies
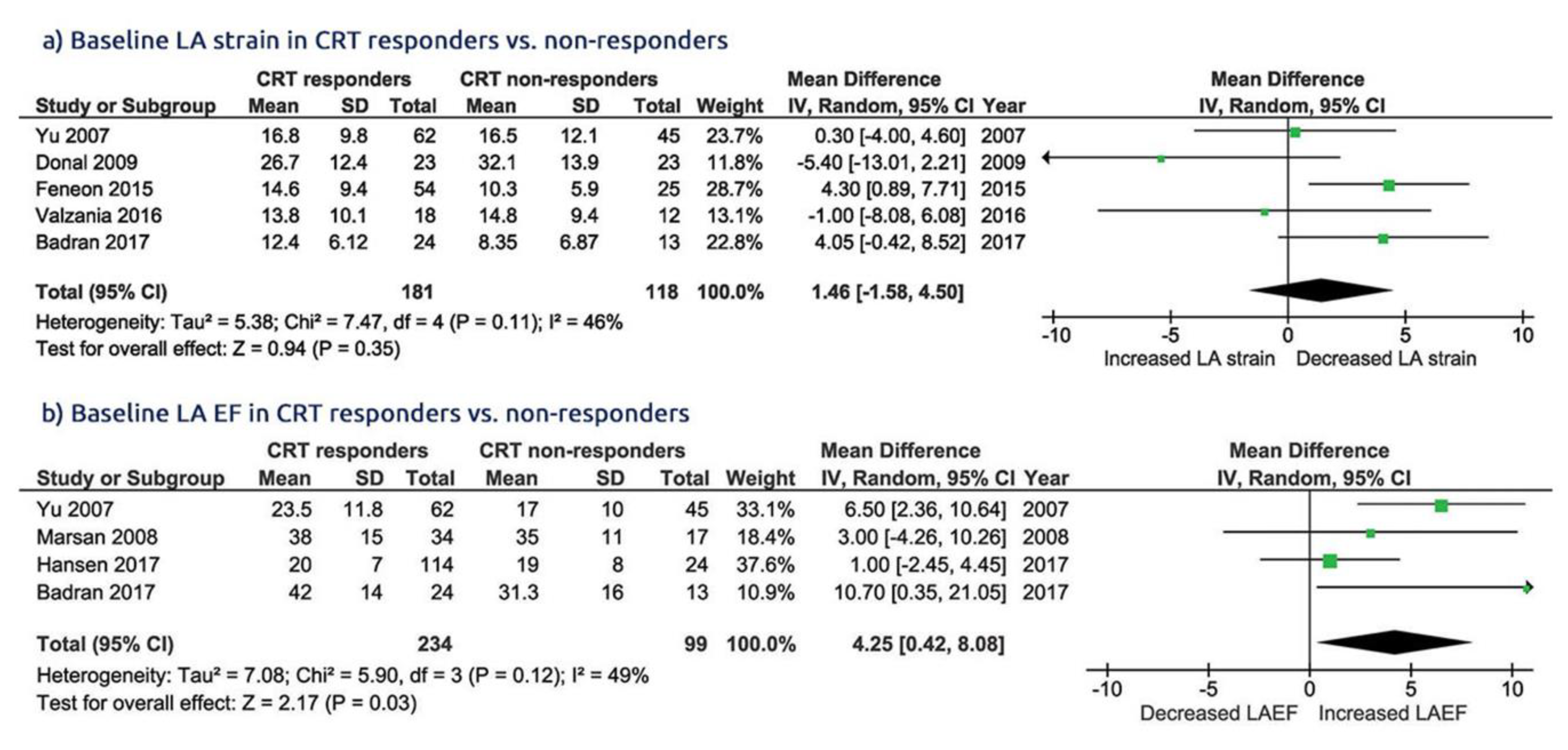
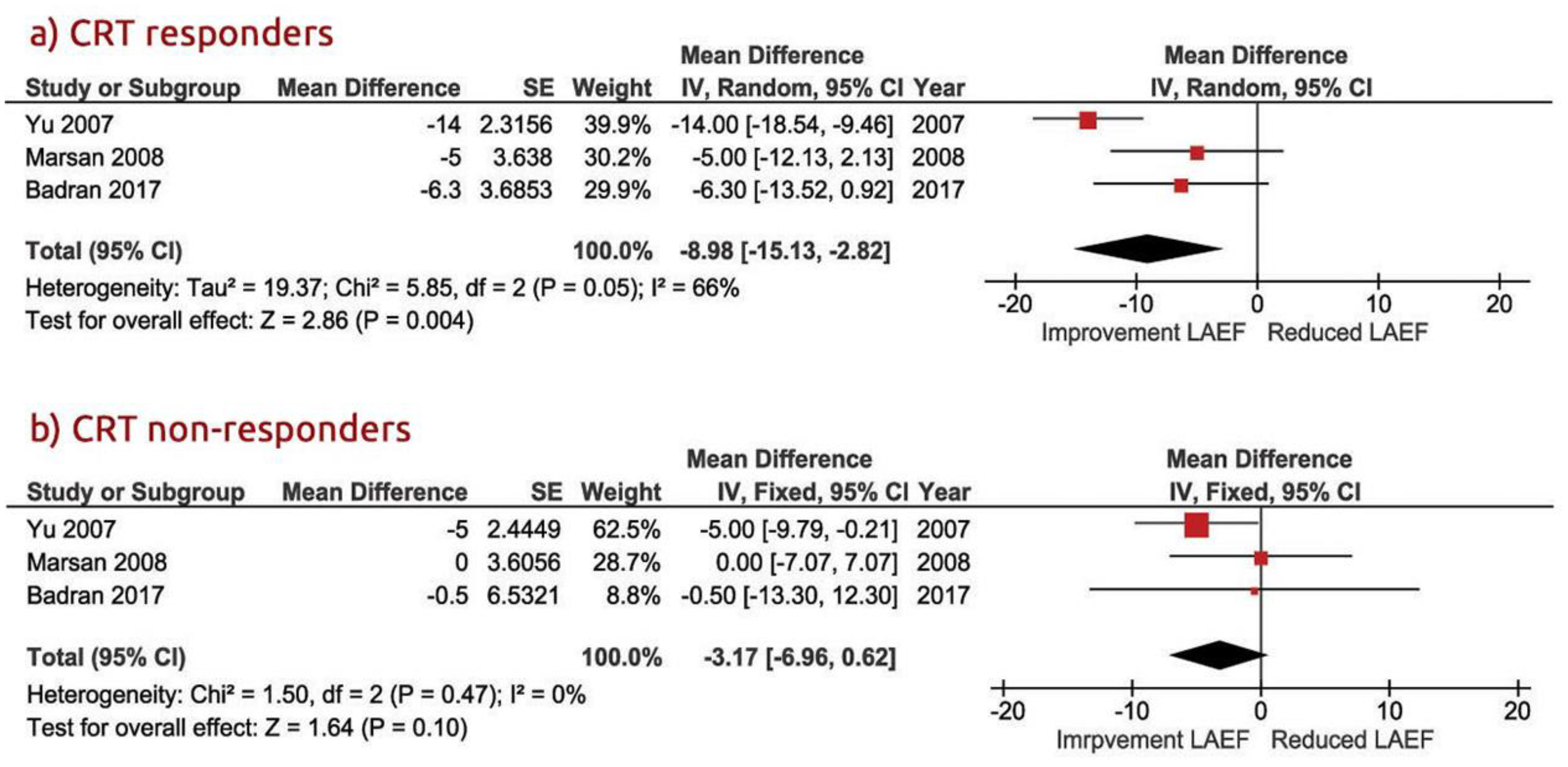
3.3. LA Function in CRT Responders Versus CRT Non-Responders
3.4. LV Dimension and Function in CRT Responders Versus CRT Non-Responders
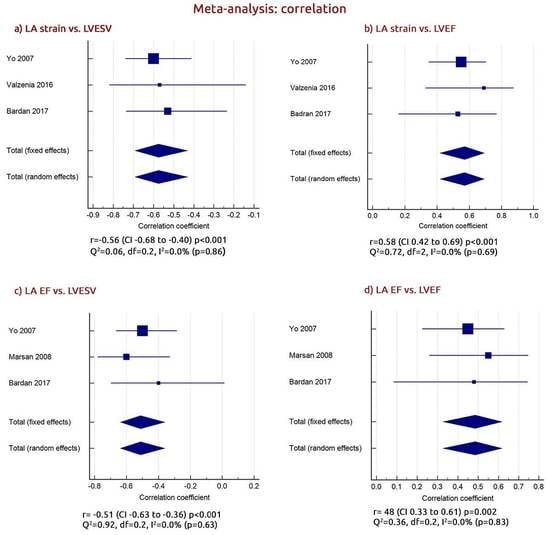
3.5. The Relationship between LA and LV Function in CRT Responders
3.6. Relationship between LA Strain Change and Baseline Age and Male Gender
3.7. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRT | Cardiac Resynchronization Therapy |
| LA | Left atrium/atrial |
| LA function | Left atrial function |
| LA EF | Left atrial emptying fraction |
| LA strain | Left atrial strain |
| LV | Left ventricle/ventricular |
| EF | Ejection fraction |
| ESV | End systolic volume |
| EDV | End diastolic volume |
| EDD | End diastolic dimension |
| ESD | End systolic dimension |
| NYHA class | New York Heart Association class |
| 2D | Two dimensional |
| 2D STE | Two-dimensional speckle-tracking echocardiography |
| PALS | Peak atrial longitudinal strain (peak atrial longitudinal strain during LV systole) |
| HF | Heart failure |
| FFrEF | Heart failure with reduced ejection fraction |
| CI | Confidence interval |
| WMD | Waited mean difference |
| SE | Standard error |
| WMD | Weighted mean difference |
References
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding non-responders to cardiac resynchronization therapy: A practical guide. Eur. Heart J. 2017, 38, 1463–1472. [Google Scholar] [CrossRef]
- Engels, E.B.; Vis, A.; van Rees, B.D.; Marcantoni, L.; Zanon, F.; Vernooy, K.; Prinzen, F.W. Improved acute haemodynamic response to cardiac resynchronization therapy using multipoint pacing cannot solely be explained by better resynchronization. J. Electrocardiol. 2018, 51, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, M.; Leclercq, C.; Lunati, M.; Landolina, M.; Auricchio, A.; Santini, M.; Boriani, G.; Lamp, B.; Proclemer, A.; Curnis, A.; et al. Cardiac resynchronization therapy in patients with atrial fibrillation: The CERTIFY study (Cardiac Resynchronization Therapy in Atrial Fibrillation Patients Multinational Registry). JACC Heart Fail. 2013, 1, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Bajraktari, G.; Henein, M.Y. The relationship between left atrial volume indexed and cardiac resynchronization therapy: A systematic review and meta-analysis. Arch. Med. Sci. 2019, 15, 845–856. [Google Scholar]
- Sarvari, S.I.; Haugaa, K.H.; Stokke, T.M.; Ansari, H.Z.; Leren, I.S.; Hegbom, F.; Smiseth, O.A.; Edvardsen, T. Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 660–667. [Google Scholar] [CrossRef]
- Ziaeian, B.; Zhang, Y.; Albert, N.M.; Curtis, A.B.; Gheorghiade, M.; Heywood, J.T.; Mehra, M.R.; O’Connor, C.M.; Reynolds, D.; Walsh, M.N.; et al. Clinical effectiveness of CRT and ICD therapy in heart failure patients by racial/ethnic classification: Insights from the IMPROVE HF registry. J. Am. Coll. Cardiol. 2014, 64, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Kosztin, A.; Boros, A.M.; Geller, L.; Merkely, B. Cardiac resynchronization therapy: Current benefits and pitfalls. Kardiol Pol. 2018, 76, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2; The Cochrane Collaboration: Charity, UK, 2009. [Google Scholar]
- Bytyçi, I.; Bajraktari, G.; Lindqvist, P.; Henein, M.Y. Compromised left atrial function and increased size predict raised cavity pressure: A systematic review and meta-analysis. Clin. Physiol. Funct. Imaging 2019, 39, 297–307. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, S.C.; Adams, J.L.; Suttorp, M.J.; Shekelle, P.G. Meta-Regression Approaches: What, Why, When, and How? Report No.: 04-0033; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004.
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.M.; Fang, F.; Zhang, Q.; Yip, G.W.; Li, C.M.; Chan, J.Y.; Wu, L.; Fung, J.W. Improvement of atrial function and atrial reverse remodeling after cardiac resynchronization therapy for heart failure. J. Am. Coll. Cardiol. 2007, 50, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsan, N.A.; Bleeker, G.B.; Ypenburg, C.; Van Bommel, R.J.; Ghio, S.; Van de Veire, N.R.; Delgado, V.; Holman, E.R.; van der Wall, E.E.; Schalij, M.J.; et al. Real-time three-dimensional echocardiography as a novel approach to assess left ventricular and left atrium reverse remodeling and to predict response to cardiac resynchronization therapy. Heart Rhythm. 2008, 5, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Tan, K.; Leclercq, C.; Ollivier, R.; Derumeaux, G.; Bernard, M.; de Place, C.; Mabo, P.; Daubert, J.C. Left atrial reverse remodeling and cardiac resynchronization therapy for chronic heart failure patients in sinus rhythm. J. Am. Soc. Echocardiogr. 2009, 22, 1152–1158. [Google Scholar] [CrossRef]
- Feneon, D.; Behaghel, A.; Bernard, A.; Fournet, M.; Mabo, P.; Daubert, J.C.; Leclercq, C.; Donal, E. Left atrial function, a new predictor of response to cardiac resynchronization therapy? Heart Rhythm. 2015, 12, 1800–1806. [Google Scholar] [CrossRef]
- Valzania, C.; Gadler, F.; Boriani, G.; Rapezzi, C.; Eriksson, M.J. Effect of Cardiac Resynchronization Therapy on Left Atrial Size and Function as Expressed by Speckle Tracking 2-Dimensional Strain. Am. J. Cardiol. 2016, 118, 237–243. [Google Scholar] [CrossRef]
- Badran, H.A.; Abdelhamid, M.A.; Ibrahim, M.T.; Abdelmoteleb, A.M.; Zarif, J.K. Left atrium in cardiac resynchronization therapy: Active participant or innocent bystander. J. Saudi Heart Assoc. 2017, 29, 259–269. [Google Scholar] [CrossRef]
- Hansen, P.B.; Sommer, A.; Nørgaard, B.L.; Kronborg, M.B.; Nielsen, J.C. Left atrial size and function as assessed by computed tomography in cardiac resynchronization therapy: Association to echocardiographic and clinical outcome. Int. J. Cardiovasc. Imaging 2017, 33, 917–925. [Google Scholar] [CrossRef]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [PubMed] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018, 15, e190–e252. [Google Scholar] [PubMed] [Green Version]
- Leon, A.R.; Greenberg, J.M.; Kanuru, N.; Baker, C.M.; Mera, F.V.; Smith, A.L.; Langberg, J.J.; DeLurgio, D.B. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: Effect of upgrading to biventricular pacing after chronic right ventricular pacing. J. Am. Coll. Cardiol. 2002, 39, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, G.A.; Choudhry, N.K.; Auricchio, A.; Ruskin, J.; Singh, J.P. Cardiac resynchronization in patients with atrial fibrillation: A meta-analysis of prospective cohort studies. J. Am. Coll. Cardiol. 2008, 52, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Riegler, L.; Salerno, G.; Limongelli, G.; Di Salvo, G.; Calabrò, P.; Del Viscovo, L.; et al. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: A two-dimensional speckle strain study. Eur. Heart J. 2007, 28, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Dernellis, J.; Toutouzas, P. A clinical appraisal of left atrial function. Eur. Heart J. 2001, 22, 22–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Ruiz, L.A.; Madrid-Miller, A.; Martínez-Flores, J.E.; González-Hermosillo, J.A.; Arenas-Fonseca, J. Left atrial longitudinal strain by speckle tracking as independent predictor of recurrence after electrical cardioversion in persistent and long standing persistent non-valvular atrial fibrillation. Int. J. Cardiovasc. Imaging 2019, 35, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Lumens, J.; Sohaib, S.M.A.; Finegold, J.A.; Kanagaratnam, P.; Tanner, M.; Duncan, E.; Moore, P.; Leyva, F.; Frenneaux, M.; et al. Cardiac resynchronization therapy: Mechanisms of action and scope for further improvement in cardiac function. Europace 2017, 19, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Lupu, S.; Mitre, A.; Sus, I.; Rudzik, R.; Beke, I.; Dobreanu, D. Changes in left atrial size and function early after cardiac resynchronization therapy as assessed by conventional two-dimensional echocardiography. Med. Ultrason. 2018, 20, 362–370. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G.; Ibrahimi, P.; Berisha, G.; Rexhepaj, N.; Henein, M.Y. Left atrial emptying fraction predicts limited exercise performance in heart failure patients. Int. J. Cardiol. Heart Vessel. 2014, 4, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Sade, L.E.; Atar, I.; Özin, B.; Yüce, D.; Müderrisoğlu, H. Determinants of New-Onset Atrial Fibrillation in Patients Receiving CRT: Mechanistic Insights From Speckle Tracking Imaging. JACC Cardiovasc. Imaging 2016, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, A.; Rossi, L.; Franchi, F.; Piepoli, M.F.; Malavasi, V.; Casali, E.; Modena, M.G.; Villani, G.Q. Effect of cardiac resynchronization therapy on left atrial reverse remodeling: Role of echocardiographic AV delay optimization. Int. J. Cardiol. 2013, 167, 1456–1460. [Google Scholar] [CrossRef]
- Blume, G.G.; Mcleod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011, 12, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P.P.; Korinek, J.; Belohlavek, M.; Narula, J.; Vannan, M.A.; Jahangir, A.; Khandheria, B.K. Left ventricular structure and function: Basic science for cardiac imaging. J. Am. Coll. Cardiol. 2006, 48, 1988–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Landman, S.R.; Stadler, R.W. Reasons for Loss of Cardiac Resynchronization Therapy Pacing. Circ. Arrhythmia Electrophysiol. 2012, 5, 884–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Study, Year | Study | Type of | Inclusion | Exclusion | Key | Echo- | Criteria for | Follow |
|---|---|---|---|---|---|---|---|---|
| Design | Intervention | Criteria | Criteria | Endpoints | Cardiography | CRT Respond | Up | |
| Yo et al. 2007 | Prospective | CRT | LV < 40% | Patients with | LA | 2DE | LVESV ≥ 15% | 3 mo |
| observational | QRS ≥ 120 ms | AF | predictors | |||||
| NYHA-III-IV | ||||||||
| Marsan et al. 2008 | Prospective | CRT-D | LV ≤ 35% | Patients with | LA and LV | 3DE | LVESV ≥ 15% | 6 mo |
| observational | QRS ≥ 120 ms | AF | predictors | |||||
| NYHA-III-IV | ||||||||
| Donal et al. 2009 | Prospective | CRT | HFrEF | Patients with AF | LA | 2DE | LVESV ≥ 15% | 6 mo |
| observational | LV ≤ 35% | Fibrillation; MR | predictors | |||||
| QRS ≥ 120 ms | (EROA > 20 mm2) | |||||||
| Feneon et al. 2015 | Prospective | CRT | LV ≤ 35% | Patients with | LA | 2DE | LVESV ≥ 15% | 6 mo |
| observational | QRS ≥ 120 ms | AF | predictors | |||||
| NYHA-II-IV | ||||||||
| NYHA-III-IV | ||||||||
| Valzania et al. 2016 | Prospective | CRT | HFrEF | Patients with | LA | 2DE | LVESV ≥ 15% | 12 mo |
| observational | LV ≤ 35% | AF | predictors | |||||
| QRS ≥ 120 ms | ||||||||
| Badran et al. 2017 | Prospective | CRT | LV ≤ 35% | Patients with | LA | 2DE | LVESV ≥ 15% | 3 mo |
| observational | QRS ≥ 120 ms | AF | predictors | |||||
| NYHA-II-IV | ||||||||
| Hansen et al. 2017 | Clinical | CRT | LV ≤ 35% | Recently MI | LA | 2DE | LVESV ≥ 15% | 6 mo |
| trial | QRS ≥ 120 ms | CRF, contrast | predictors | |||||
| NYHA-II-IV | allergy |
| Study, Year | Arms | No. | Age | Male | QRS | NYHA | Ischemic | Mean | Mean |
|---|---|---|---|---|---|---|---|---|---|
| Year | (%) | Duration (ms) | Functional Class | Etiology (%) | Change of LA Strain % | Change of LA EF % | |||
| Yo et al. 2007 | R | 62 | 66 ± 11 * | 75 * | 142 ± 28 | 3.0 ± 0.5 | NR | −5.1 | −14 |
| Non-R | 45 | 154 ± 31 | 3.0 ± 0.4 | NR | −2.7 | −5.2 | |||
| Marsan et al. 2008 | R | 34 | 65 ± 7 | 78 | 142 ± 28 | 3.0 ± 0.5 | NR | −6 | −5.0 |
| Non-R | 17 | 67 ± 10 | 70 | 154 ± 31 | 3.0 ± 0.4 | NR | 0 | 0 | |
| Donal et al. 2009 | R | 23 | 67 ± 10.4 * | 76 * | NR | 3.2 ± 0.6 * | NR | −12.1 | NR |
| Non-R | 23 | 1.4 | NR | ||||||
| Feneon et al. 2015 | R | 54 | 62.3 ± 10 | 63 | 163 ± 27 | N−II = 24% | 18.6 | NR | NR |
| Non-R | 25 | 66.5 ± 10 | 80 | 158 ± 30 | N−II = 22% | 60 | NR | NR | |
| Valzania et al. 2016 | R | 18 | 61 ± 13 | 63 | 160 ± 24 | 2.9 ± 0.2 | 20 | −5.5 | −72 |
| Non-R | 12 | 67 ± 8 | 50 | 159 ± 23 | 3.1 ± 0.3 | 50 | 4.5 | NR | |
| Badran et al. 2017 | R | 24 | 56 ± 9.8 | 71 | NR | N−IV = 33% | 29 | −4.2 | −35.2 |
| Non-R | 13 | 53 ± 9.5 | 69 | NR | N−IV = 46% | 23 | 2.87 | −0.3 | |
| Hansen et al. 2017 | R | 114 | 69.4 ± 9 * | 80 * | 166.2 ± 23.0 * | N−IV = 3% * | 50 * | −4.4 | −5.0 |
| Non-R | 24 | −2 | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bytyçi, I.; Bajraktari, G.; Lindqvist, P.; Henein, M.Y. Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 298. https://doi.org/10.3390/jcm9020298
Bytyçi I, Bajraktari G, Lindqvist P, Henein MY. Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(2):298. https://doi.org/10.3390/jcm9020298
Chicago/Turabian StyleBytyçi, Ibadete, Gani Bajraktari, Per Lindqvist, and Michael Y. Henein. 2020. "Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 2: 298. https://doi.org/10.3390/jcm9020298
APA StyleBytyçi, I., Bajraktari, G., Lindqvist, P., & Henein, M. Y. (2020). Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(2), 298. https://doi.org/10.3390/jcm9020298