Arginase 2 Deficiency Promotes Neuroinflammation and Pain Behaviors Following Nerve Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Pain Model
2.3. Pain Behavioral Test
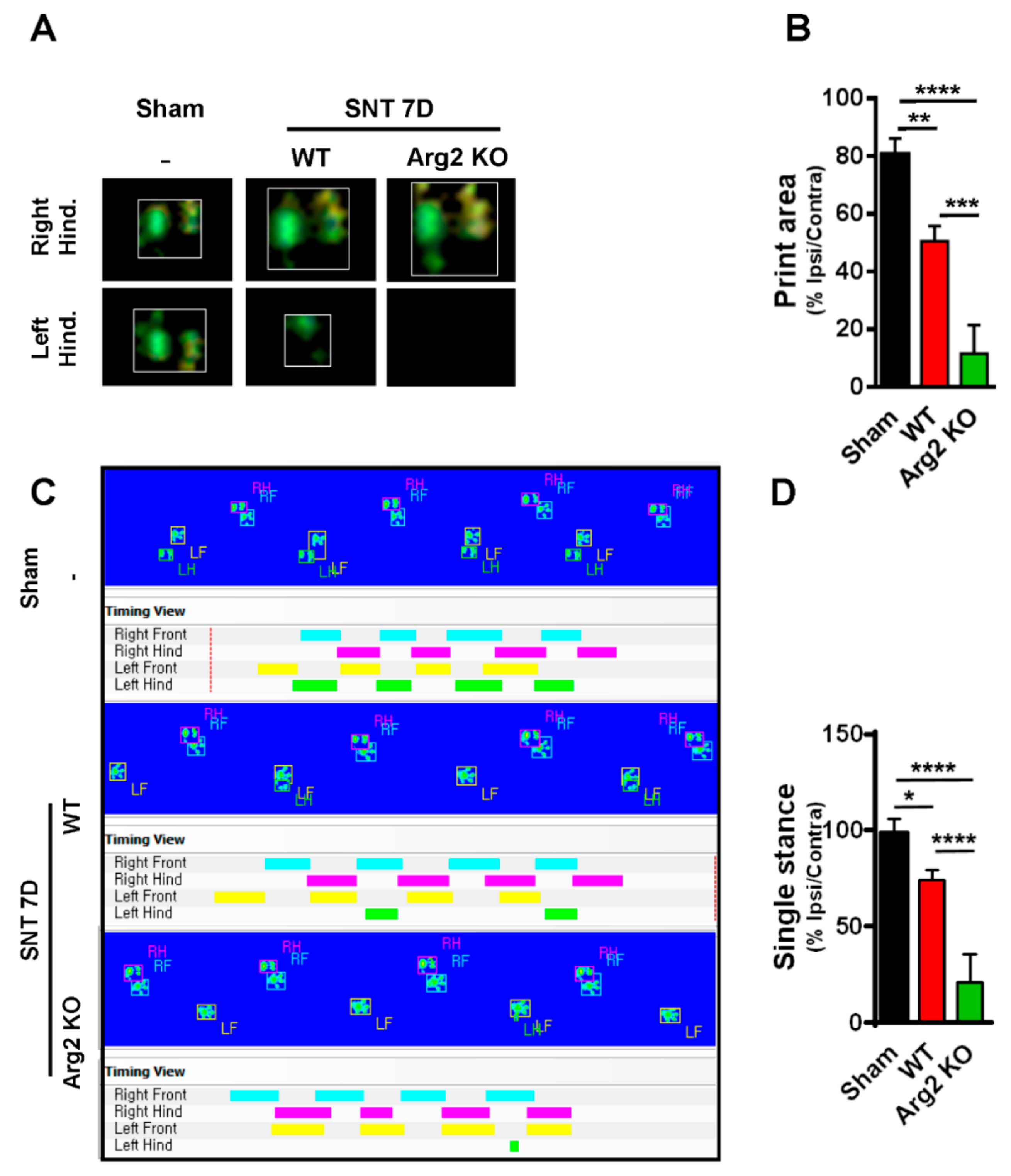
2.4. CatWalk Automated Gait Analysis in SNT Mice
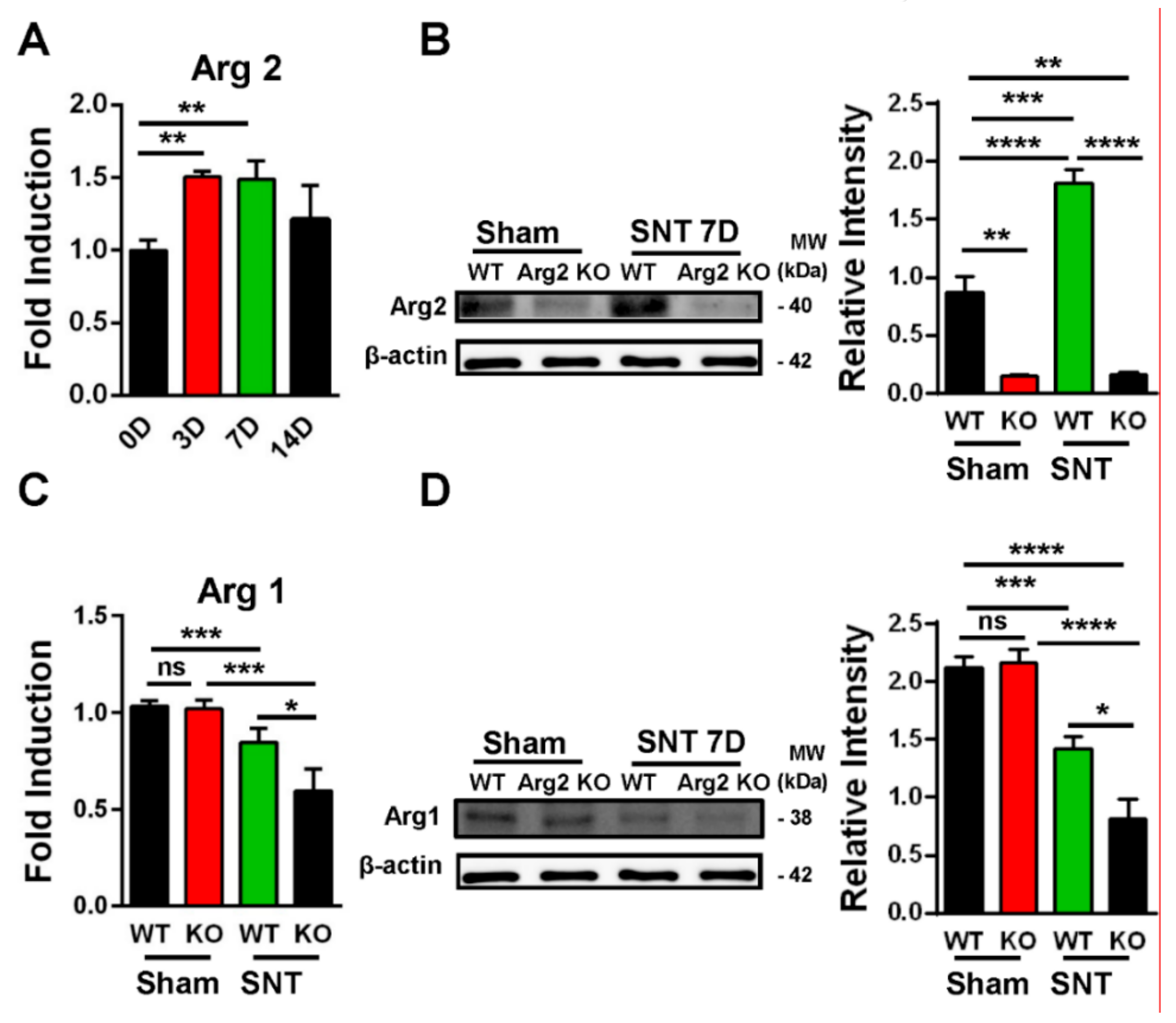
2.5. Western Blot Analysis
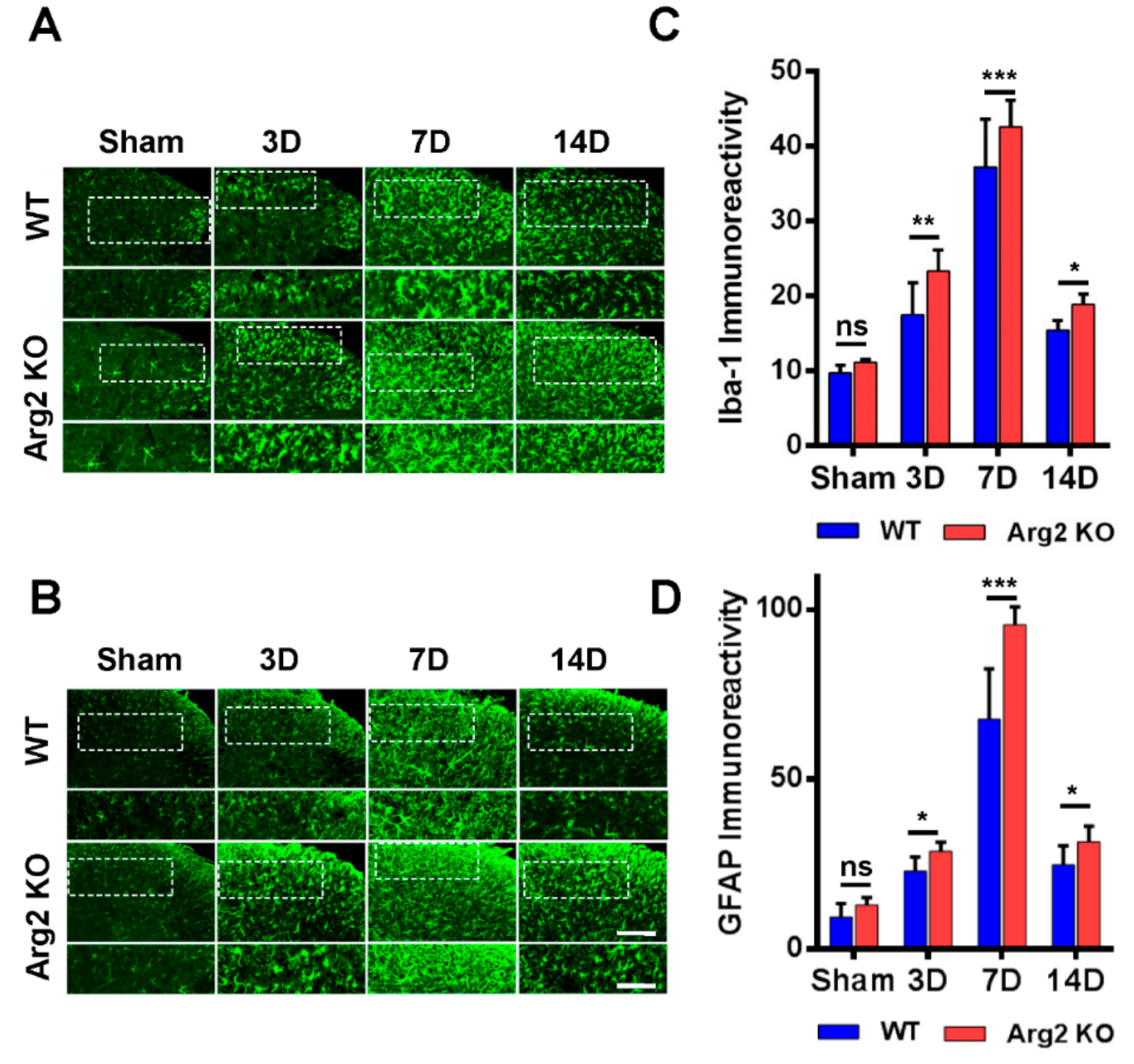
2.6. Immunostaining Analysis
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.8. Reactive Oxygen Species (ROS) Detection Assay
2.9. Statistical Analysis
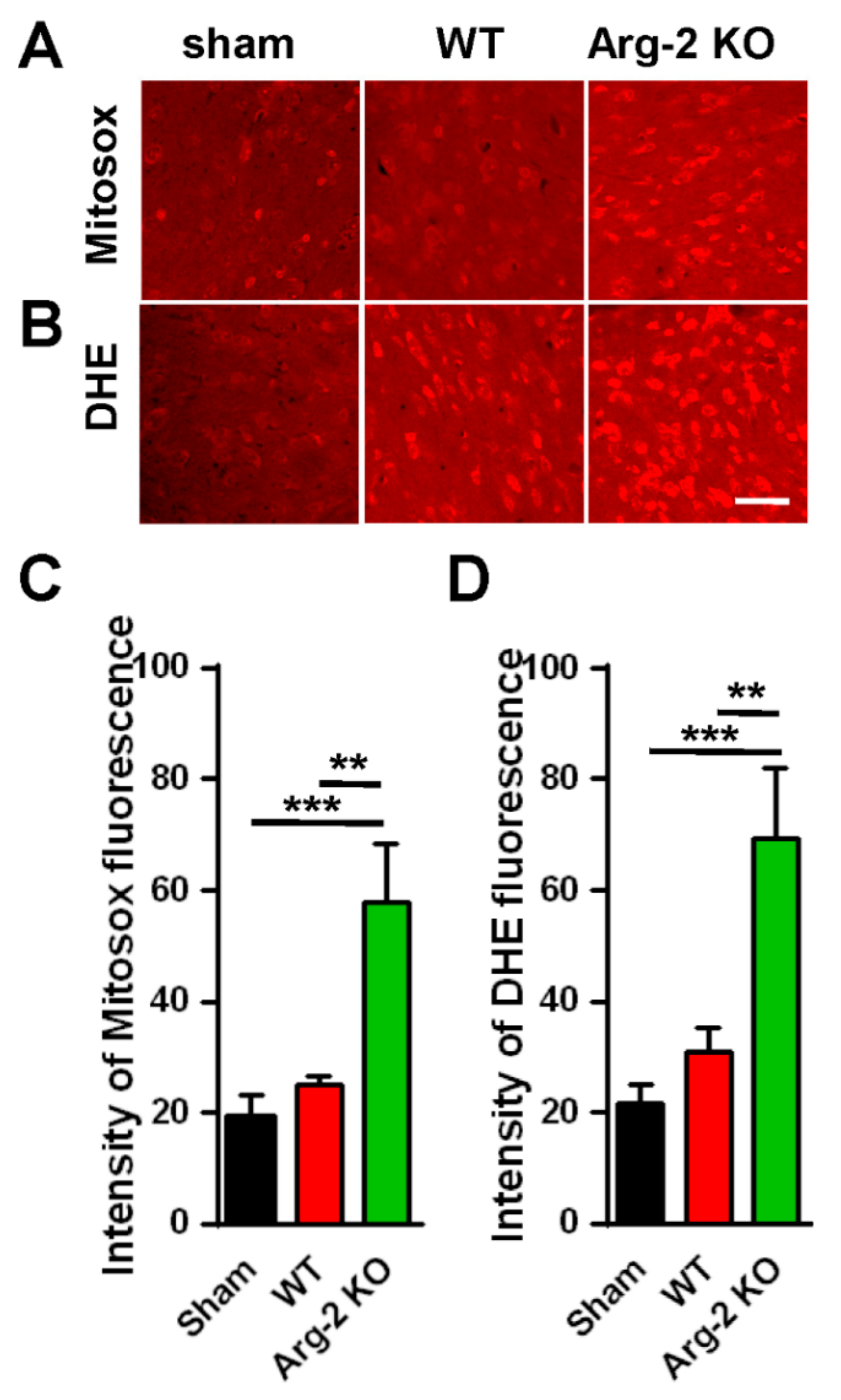
3. Results
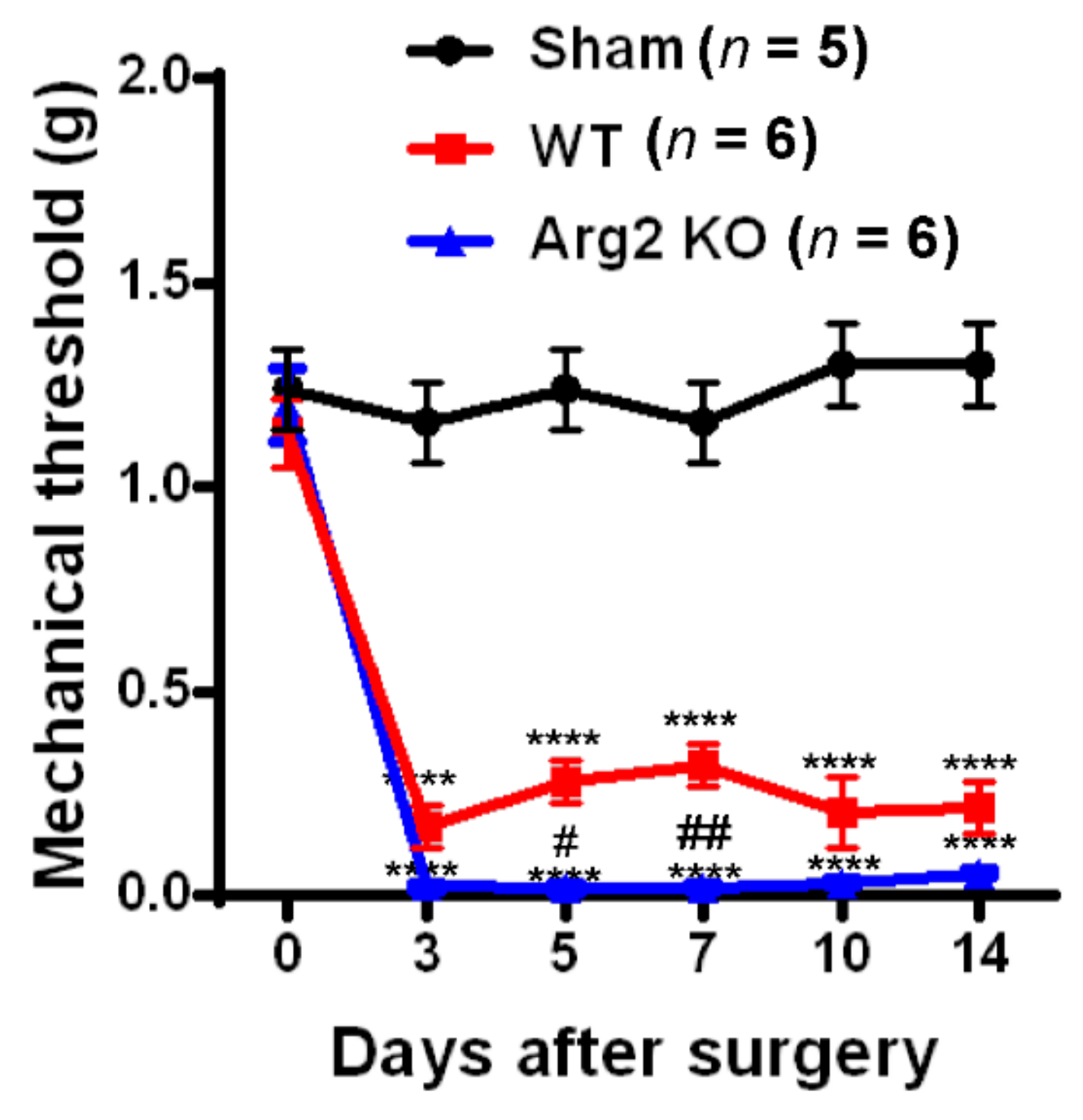
Pain Behaviors from SNT-Induced Neuropathic Pain in WT and Arg2 KO Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H. Modulation of macrophage activation and programming in immunity. J. Cell Physiol. 2013, 228, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Bertani, F.R.; Mozetic, P.; Fioramonti, M.; Iuliani, M.; Ribelli, G.; Pantano, F.; Santini, D.; Tonini, G.; Trombetta, M.; Businaro, L.; et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci. Rep. 2017, 7, 8965. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satriano, J. Arginine pathways and the inflammatory response: interregulation of nitric oxide and polyamines: review article. Amino Acids 2004, 26, 321–329. [Google Scholar] [CrossRef]
- Ash, D.E. Structure and function of arginases. J. Nutr. 2004, 134, 2760S–2764S. [Google Scholar] [CrossRef]
- Choi, S.; Park, C.; Ahn, M.; Lee, J.H.; Shin, T. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem. 2012, 114, 487–494. [Google Scholar] [CrossRef]
- Crombez, E.A.; Cederbaum, S.D. Hyperargininemia due to liver arginase deficiency. Mol. Genet. Metab. 2005, 84, 243–251. [Google Scholar] [CrossRef]
- Tsang, J.P.; Poon, W.L.; Luk, H.M.; Fung, C.W.; Ching, C.K.; Mak, C.M.; Lam, C.W.; Siu, T.S.; Tam, S.; Wong, V.C. Arginase deficiency with new phenotype and a novel mutation: contemporary summary. Pediatr. Neurol. 2012, 47, 263–269. [Google Scholar] [CrossRef]
- Iyer, R.K.; Yoo, P.K.; Kern, R.M.; Rozengurt, N.; Tsoa, R.; O’Brien, W.E.; Yu, H.; Grody, W.W.; Cederbaum, S.D. Mouse model for human arginase deficiency. Mol. Cell Biol. 2002, 22, 4491–4498. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014, 5, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, S.M., Jr.; Gao, T.; Cooper, T.K.; Kepka-Lenhart, D.; Awad, A.S. Arginase-2 mediates diabetic renal injury. Diabetes 2011, 60, 3015–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.S.; Yang, J.I.; Kim, W.; Kim, H.E.; Kim, S.K.; Won, Y.; Son, Y.O.; Chun, C.H.; Chun, J.S. Critical role for arginase II in osteoarthritis pathogenesis. Ann. Rheum. Dis. 2019, 78, 421–428. [Google Scholar] [CrossRef]
- Myers, R.R.; Campana, W.M.; Shubayev, V.I. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov. Today 2006, 11, 8–20. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Wu, X.; Fossett, N. Upregulation of the Drosophila Friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol. Cell Biol. 2009, 29, 6086–6096. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, H.; Tian, L.; Tong, L.; Deng, J.; Zhang, Y.; Dong, H.; Xiong, L. Sevoflurane preconditioning-induced neuroprotection is associated with Akt activation via carboxy-terminal modulator protein inhibition. Br. J. Anaesth. 2015, 114, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, A.; Deumens, R.; Scheffel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Fuhrmann, T.; Brook, G.A.; Pallua, N. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J. Neurosci. Methods 2008, 173, 91–98. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Bal-Price, A.; Brown, G.C. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001, 21, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rajaram, M.V.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Hong, Y.S.; Chun, C.M.; Moon, J.D.; Kim, S.J.; Jung, I.C.; Yoon, Y.H.; Lee, B.A.; Moon, S.W.; Choi, S.H.; et al. Anti-inflammatory effects of IL-4 and IL-10 on human polymorphonuclear leukocytes. J. Korean Med. Sci. 2002, 17, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Phạm, T.L.; Shin, J.; Shin, N.; Kang, D.-W.; Lee, S.Y.; Lee, W.; Kim, C.-S.; Kim, S.R.; Hong, J.; et al. Arginase 2 Deficiency Promotes Neuroinflammation and Pain Behaviors Following Nerve Injury in Mice. J. Clin. Med. 2020, 9, 305. https://doi.org/10.3390/jcm9020305
Yin Y, Phạm TL, Shin J, Shin N, Kang D-W, Lee SY, Lee W, Kim C-S, Kim SR, Hong J, et al. Arginase 2 Deficiency Promotes Neuroinflammation and Pain Behaviors Following Nerve Injury in Mice. Journal of Clinical Medicine. 2020; 9(2):305. https://doi.org/10.3390/jcm9020305
Chicago/Turabian StyleYin, Yuhua, Thuỳ Linh Phạm, Juhee Shin, Nara Shin, Dong-Wook Kang, Sun Yeul Lee, Wonhyung Lee, Cuk-Seong Kim, Sang Ryong Kim, Jinpyo Hong, and et al. 2020. "Arginase 2 Deficiency Promotes Neuroinflammation and Pain Behaviors Following Nerve Injury in Mice" Journal of Clinical Medicine 9, no. 2: 305. https://doi.org/10.3390/jcm9020305
APA StyleYin, Y., Phạm, T. L., Shin, J., Shin, N., Kang, D.-W., Lee, S. Y., Lee, W., Kim, C.-S., Kim, S. R., Hong, J., & Kim, D.-W. (2020). Arginase 2 Deficiency Promotes Neuroinflammation and Pain Behaviors Following Nerve Injury in Mice. Journal of Clinical Medicine, 9(2), 305. https://doi.org/10.3390/jcm9020305






