Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility
Abstract
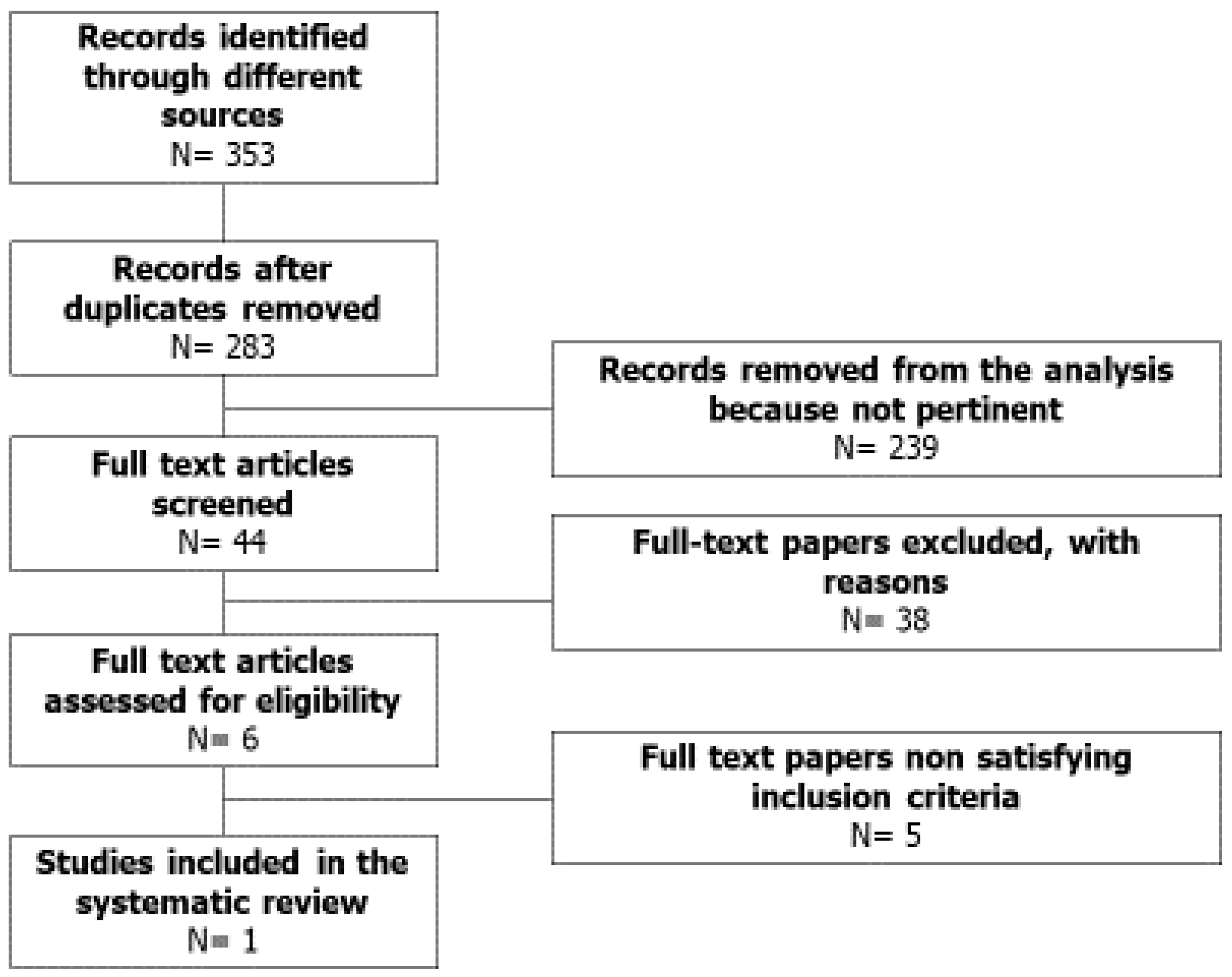
1. Introduction
2. Methods
2.1. Sources
2.2. Study Selection
- Design: randomized controlled and not-controlled clinical trial;
- Patients inclusion criteria: idiopathic asthenozoospermia
- Patients exclusion criteria: known causes of male infertility such as male accessory gland infection, varicocele, hypogonadism, Y chromosome microdeletions, testicular torsion or trauma, history of cryptorchidism, as well as thyroid, pituitary or adrenal disorders, liver or kidney failure.
- Study intervention: antioxidants administration in vivo or in vitro
- Study outcome: both sperm motility and mitochondrial membrane potential evaluated after antioxidant administration, compared to those of a treated or not-treated control group or with baseline. Spontaneous pregnancy.
3. Results
4. Sperm Mitochondria: Anatomy
5. Sperm Mitochondrial Metabolism
5.1. A Long Debate: Glycolysis or Oxidative Phosphorylation?
5.2. Reactive Oxygen Species and Sperm Mitochondria
6. Techniques to Study Sperm Mitochondria Function
7. Asthenozoospermia and Sperm Mitochondrial Dysfunction
8. Effects of Prokinetic/Antioxidant Therapy on Sperm Mitochondrial Function and Motility
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Travis, A.J.; Foster, J.A.; Rosenbaum, N.A.; Visconti, P.E.; Gerton, G.L.; Kopf, G.S.; Moss, S.B. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol. Biol. Cell 1998, 9, 263–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darr, C.R.; Cortopassi, G.A.; Datta, S.; Varner, D.D.; Meyers, S.A. Mitochondrial oxygen consumption is a unique indicator of stallion spermatozoal health and varies with cryopreservation media. Theriogenology 2016, 86, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Losano, J.D.A.; Padín, J.F.; Méndez-López, I.; Angrimani, D.S.R.; García, A.G.; Barnabe, V.H.; Nichi, M. The Stimulated Glycolytic Pathway Is Able to Maintain ATP Levels and Kinetic Patterns of Bovine Epididymal Sperm Subjected to Mitochondrial Uncoupling. Oxidat. Med. Cellular Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Zhuang, X.J.; Wei, Y.M.; Zhang, M.; Lu, S.S.; Lu, Y.Q.; Yang, X.G.; Lu, K.H. Comparison of Mitochondrial Function in Boar and Bull Spermatozoa Throughout Cryopreservation Based on JC-1 Staining. CryoLetters 2017, 38, 75–79. [Google Scholar] [PubMed]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Duca, Y.; Mongioi, L.M.; Cannarella, R.; Giacone, F.; Calogero, A.E. FSH therapy for idiopathic male infertility: Four schemes are better than one. Aging Male 2019, 3, 16. [Google Scholar] [CrossRef]
- Ankel-Simons, F.; Cummins, J.M. Misconceptions about mitochondria and mammalian fertilization: Implications for theories on human evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 13859–13863. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Lesich, K.A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef]
- Ho, H.C.; Wey, S. Three dimensional rendering of the mitochondrial sheath morphogenesis during mouse spermiogenesis. Microsc. Res. Tech. 2007, 70, 719–723. [Google Scholar] [CrossRef]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohé, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Focarelli, R.; Piomboni, P.; Coppola, L.; Zara, V. Oxygen uptake by mitochondria in demembranated human spermatozoa: A reliable tool for the evaluation of sperm respiratory efficiency. Int. J. Androl. 2008, 31, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Jaradat, S.; Grossman, L.I. Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb—The counterpart to testes-specific cytochrome c? Mol. Reprod. Dev. 2003, 66, 8–16. [Google Scholar] [CrossRef]
- Gerez de Burgos, N.M.; Gallina, F.; Burgos, C.; Blanco, A. Effect of L-malate on pyruvate dehydrogenase activity of spermatozoa. Arch. Biochem. Biophys. 1994, 308, 520–524. [Google Scholar]
- Huszar, G.; Stone, K.; Dix, D.; Vigue, L. Putative creatine kinase M-isoform in human sperm is identifiedas the 70-kilodalton heat shock protein HspA2. Biol. Reprod. 2000, 63, 925–932. [Google Scholar] [CrossRef]
- Peterson, R.N.; Freund, M. Profile of glycolytic enzyme activities in human spermatozoa. Fertil. Steril. 1970, 21, 151–158. [Google Scholar] [CrossRef]
- Peterson, R.N.; Freund, M. Citrate formation from exogenous substrates by washed human spermatozoa. J. Reprod. Fertil. 1974, 38, 73–79. [Google Scholar] [CrossRef][Green Version]
- Storey, B.T.; Kayne, F.J. Energy metabolism of spermatozoa. VII. Interactions between lactate, pyruvate and malate as oxidative substrates for rabbit sperm mitochondria. Biol. Reprod. 1978, 18, 527–536. [Google Scholar] [CrossRef]
- Williams, A.C.; Ford, W.C. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar]
- Auger, J.; Ronot, X.; Dadoune, J.P. Human sperm mitochondrial function related to motility: A flow and image cytometric assessment. J. Androl. 1989, 10, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Leonce, S.; Jouannet, P.; Ronot, X. Flow cytometric sorting of living, highly motile human spermatozoa based on evaluation of their mitochondrial activity. J. Histochem. Cytochem. 1993, 41, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.Y.; Garner, D.L.; Bruns, E.S.; Ericsson, S.A.; Prins, G.S. Comparison of motility and flow cytometric assessments of seminal quality in fresh, 24-h extended and cryopreserved human spermatozoa. J. Androl. 1993, 14, 374–384. [Google Scholar] [PubMed]
- Ruiz-Pesini, E.; Diez, C.; Lapeña, A.C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Arenas, J.; López-Pérez, M.J. Correlation of sperm motility with mitochondrial enzymatic activities. Clin. Chem. 1998, 44, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Lapeña, A.C.; Díez, C.; Alvarez, E.; Enríquez, J.A.; López-Pérez, M.J. Seminal quality correlates with mitochondrial functionality. Clin. Chim. Acta 2000, 300, 97–105. [Google Scholar] [CrossRef]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Schulz, M.A.; Sánchez, R.; Villegas, J.V. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top Dev. Biol. 2007, 77, 3–19. [Google Scholar]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and Protein Synthesis in Mitochondria Enhance the Duration of High-Speed Linear Motility in Boar Sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Duan, C.; Willis, W.D.; Goulding, E.H.; Kung, A.; Eddy, E.M.; Goldberg, E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008, 79, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Griveau, J.E.; Renard, P.; Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int. J. Androl. 1994. [Google Scholar] [CrossRef]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef]
- Kodama, H.; Yamaguchi, R.; Fukuda, J.; Kasai, H.; Tanaka, T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil. Steril. 1997, 68, 519–524. [Google Scholar] [CrossRef]
- Losano, J.D.A.; Angrimani, D.S.R.; Ferreira Leite, R.; Simões da Silva, B.D.C.; Barnabe, V.H.; Nichi, M. Spermatic mitochondria: Role in oxidative homeostasis, sperm function and possible tools for their assessment. Zygote 2018, 26, 251–260. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef]
- Garner, D.L.; Thomas, C.A.; Joerg, H.W.; DeJarnette, J.M.; Marshall, C.E. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biol. Reprod. 1997, 57, 1401–1406. [Google Scholar] [CrossRef]
- Piccoli, C.; Scrima, R.; D’Aprile, A.; Ripoli, M.; Lecce, L.; Boffoli, D.; Capitanio, N. Mitochondrial dysfunction in hepatitis C virus infection. Biochim. Biophys. Acta 2006, 1757, 1429–1437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Evans, G.; Maxwell, W.M. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology 2005, 63, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.K.; Kunze, E.; Hammerstedt, R.H. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function using flow cytometry. Biol. Reprod. 1990, 43, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Thomas, C.A. Organelle-specific probe JC-1 identifies membrane potential differences in the mitochondrial function of bovine sperm. Mol. Reprod. Dev. 1999, 53, 222–229. [Google Scholar] [CrossRef]
- Gravance, C.G.; Garner, D.L.; Baumber, J.; Ball, B.A. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology 2000, 53, 1691–1703. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Johannisson, A.; Gil, J.; Kaabi, M.; Anel, L.; Paz, P.; Rodriguez-Martinez, H. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim. Reprod. Sci. 2004, 84, 121–133. [Google Scholar] [CrossRef]
- Cheuquemán, C.; Bravo, P.; Treulén, F.; Giojalas, L.; Villegas, J.; Sánchez, R.; Risopatrón, J. Sperm membrane functionality in the dog assessed by flow cytometry. Reprod. Domest. Anim. 2012, 47, 39–43. [Google Scholar] [CrossRef]
- Cheuquemán, C.; Merino, O.; Giojalas, L.; Von Baer, A.; Sánchez, R.; Risopatrón, J. Assessment of sperm function parameters and DNA fragmentation in ejaculated alpaca sperm (Lama pacos) by flow cytometry. Reprod. Domest. Anim. 2013, 48, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Lugli, E.; Troiano, L.; Cossarizza, A. Polychromatic analysis of mitochondrial membrane potential using JC-1. Curr. Protoc. Cytom. 2007. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Ramalho-Santos, J.; St John, J.C. The expression of polymerase gamma and mitochondrial transcription factor A and the regulation of mitochondrial DNA content in mature human sperm. Hum. Reprod. 2007, 22, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Villegas, J.V.; Boguen, R.; Treulen, F.; Sánchez, R.; Mallmann, P.; Isachenko, V.; Rahimi, G.; Isachenko, E. Use of the fluorescent dye tetramethylrhodamine methyl ester perchlorate for mitochondrial membrane potential assessment in human spermatozoa. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Evenson, D.P.; Darzynkiewicz, Z.; Melamed, M.R. Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. J. Histochem. Cytochem. 1982, 30, 279–280. [Google Scholar] [CrossRef]
- Wang, X.; Sharma, R.K.; Gupta, A.; George, V.; Thomas, A.J.; Falcone, T.; Agarwal, A. Alterations in mitochondria membrane potential and oxidative stress in infertile men: A prospective observational study. Fertil. Steril. 2003, 80, 844–850. [Google Scholar] [CrossRef]
- Troiano, L.; Granata, A.R.; Cossarizza, A.; Kalashnikova, G.; Bianchi, R.; Pini, G.; Tropea, F.; Carani, C.; Franceschi, C. Mitochondrial membrane potential and DNA stainability in human sperm cells: A flow cytometry analysis with implications for male infertility. Exp. Cell Res. 1998, 241, 384–393. [Google Scholar] [CrossRef]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J. Androl. 2002, 4, 97–103. [Google Scholar]
- Zhang, G.; Wang, Z.; Ling, X.; Zou, P.; Yang, H.; Chen, Q.; Zhou, N.; Sun, L.; Gao, J.; Zhou, Z.; et al. Mitochondrial biomarkers reflect semen quality: Results from the MARCHS study in Chongqing, China. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Piasecka, M.; Kawiak, J. Sperm mitochondria of patients with normal sperm motility and with asthenozoospermia: Morphological and functional study. Folia. Histochem. Cytobiol. 2003, 41, 125–139. [Google Scholar]
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Paiva, C.; Attardo Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of proteins involved in human sperm motility using high throughput differential proteomics. J. Proteome. Res. 2014, 13, 5670–5684. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Aversa, A.; La Vignera, S.; Corona, G.; Ferlin, A. The use of nutraceuticals in male sexual and reproductive disturbances: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Invest. 2017, 40, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radical. Biology. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Raigani, M.; Yaghmaei, B.; Amirjannti, N.; Lakpour, N.; Akhondi, M.M.; Zeraati, H.; Hajihosseinal, M.; Sadeghi, M.R. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia 2014, 46, 956–962. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Vaezi, G.; Shariatzadeh, M.A.; Malekirad, A.A. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- González-Ravina, C.; Aguirre-Lipperheide, M.; Pinto, F.; Martín-Lozano, D.; Fernández-Sánchez, M.; Blasco, V.; Santamaría-López, E.; Candenas, L. Effect of dietary supplementation with a highly pure and concentrated docosahexaenoic acid (DHA) supplement on human sperm function. Reprod. Biol. 2018, 18, 282–288. [Google Scholar] [CrossRef]
- Colone, M.; Marelli, G.; Unfer, V.; Bozzuto, G.; Molinari, A.; Stringaro, A. Inositol activity in oligoasthenoteratospermia—An in vitro study. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 891–896. [Google Scholar]
- Rubino, P.; Palini, S.; Chigioni, S.; Carlomagno, G.; Quagliariello, A.; De Stefani, S.; Baglioni, A.; Bulletti, C. Improving fertilization rate in ICSI cycles by adding myoinositol to the semen preparation procedures: A prospective, bicentric, randomized trial on sibling oocytes. J. Assist. Reprod. Genet. 2015, 32, 387–394. [Google Scholar] [CrossRef][Green Version]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 109–112. [Google Scholar] [CrossRef]
- Calogero, A.E.; Gullo, G.; La Vignera, S.; Condorelli, R.A.; Vaiarelli, A. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: A prospective double-blind randomized placebo-controlled study. Andrology 2015, 3, 491–495. [Google Scholar] [CrossRef]
- Palmieri, M.; Papale, P.; Della Ragione, A.; Quaranta, G.; Russo, G.; Russo, S. Corrigendum to “In Vitro Antioxidant Treatment of Semen Samples in Assisted Reproductive Technology: Effects of Myo-Inositol on Nemaspermic Parameters”. Int. J. Endocrinol. 2017. [Google Scholar] [CrossRef]
- Capece, M.; Romeo, G.; Ruffo, A.; Romis, L.; Mordente, S.; Di Lauro, G. A phytotherapic approach to reduce sperm DNA fragmentation in patients with male infertility. Urologia 2017, 84, 79–82. [Google Scholar] [CrossRef]
- Canepa, P.; Dal Lago, A.; De Leo, C.; Gallo, M.; Rizzo, C.; Licata, E.; Anserini, P.; Rago, R.; Scaruffi, P. Combined treatment with myo-inositol, alpha-lipoic acid, folic acid and vitamins significantly improves sperm parameters of sub-fertile men: A multi-centric study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7078–7085. [Google Scholar] [CrossRef]
| Authors | Method | Results | |
|---|---|---|---|
| Mitochondria | Sperm Motility | ||
| Everson et al., 1982 [55] | Determination of MMP with Rhodamine 123 | Reduced MMP | Asthenozoospermia |
| Ruiz-Pesini et al., 1998 [24] | Determination of enzymatic activity by spectrophotometric assay | Reduced activity of Complexes I, II and IV and of citrate synthase | Asthenozoospermia |
| Ruiz-Pesini et al., 2000 [25] | Determination of enzymatic activity by spectrophotometric assay | Reduced activity of Complexes I, II, I+III, II+IV and IV and of citrate synthase | Asthenozoospermia |
| Troiano et al., 1998 [57] | Determination of MMP with JC-1 | Reduced MMP | Asthenozoospermia |
| Marchetti et al., 2002 [26] | Determination of MMP | High MMP and low DNA fragmentation | High motility |
| Kasai et al., 2002 [58] | Determination of MMP with JC-1 | High MMP | High motility |
| Wang et al., 2003 [56] | Determination of MMP with DiOC6(3) and ROS with chemiluminescence assay using luminol. | Reduced MMP Higher ROS levels | Asthenozoospermia |
| Piasecka et al., 2003 [60] | Determination of MMP with JC-1 and Mito Tracker Green FM | Two subpopulations, one with a low and the other with a high MMP | Asthenozoospermia |
| Gallon et al., 2006 [27] | Determination of MMP with DiOC6(3) | High MMP | High motility |
| Ferramosca et al., 2008 [13] | Determination of oxygen consumption by polarographic assay | Reduction of respiratory control ratio | Asthenozoospermia |
| Paoli et al., 2011 [46] | Determination of MMP with JC-1 | Increasing MMP Low MMP | 13 motility classes (from 0–60%) with an increment of 5% between classes Immotility or severe asthenozoospermia |
| Pelliccione et al., 2011 [61] | Analysis of tail middle piece (MP) by transmission electron microscopy | Structural defects in mitochondrial membranes | Asthenozoospermia |
| Zhang et al., 2016 [59] | Determination of MMP with JC-1; mitochondrial DNA copy number (mtDNAcn), mtDNA integrity analysis using long PCR and apoptotic parameters | High MMP Low mtDNAcn High mtDNA integrity | High motility |
| Amaral et al., 2014 [62] | Proteomic study | Dysregulation of mitochondrial proteins | Asthenozoospermia |
| Nowicka-Bauer et al., 2018 [63] | Proteomic data supported by two additional mitochondrial tests (JC-1 and MitoSox Red) | Dysregulation of mitochondrial proteins | Isolated Asthenozoospermia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. https://doi.org/10.3390/jcm9020363
Barbagallo F, La Vignera S, Cannarella R, Aversa A, Calogero AE, Condorelli RA. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. Journal of Clinical Medicine. 2020; 9(2):363. https://doi.org/10.3390/jcm9020363
Chicago/Turabian StyleBarbagallo, Federica, Sandro La Vignera, Rossella Cannarella, Antonio Aversa, Aldo E. Calogero, and Rosita A. Condorelli. 2020. "Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility" Journal of Clinical Medicine 9, no. 2: 363. https://doi.org/10.3390/jcm9020363
APA StyleBarbagallo, F., La Vignera, S., Cannarella, R., Aversa, A., Calogero, A. E., & Condorelli, R. A. (2020). Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. Journal of Clinical Medicine, 9(2), 363. https://doi.org/10.3390/jcm9020363









