Opposite Roles of BAP1 in Overall Survival of Uveal Melanoma and Cutaneous Melanoma
Abstract
:1. Introduction
2. Experimental Section
3. Results
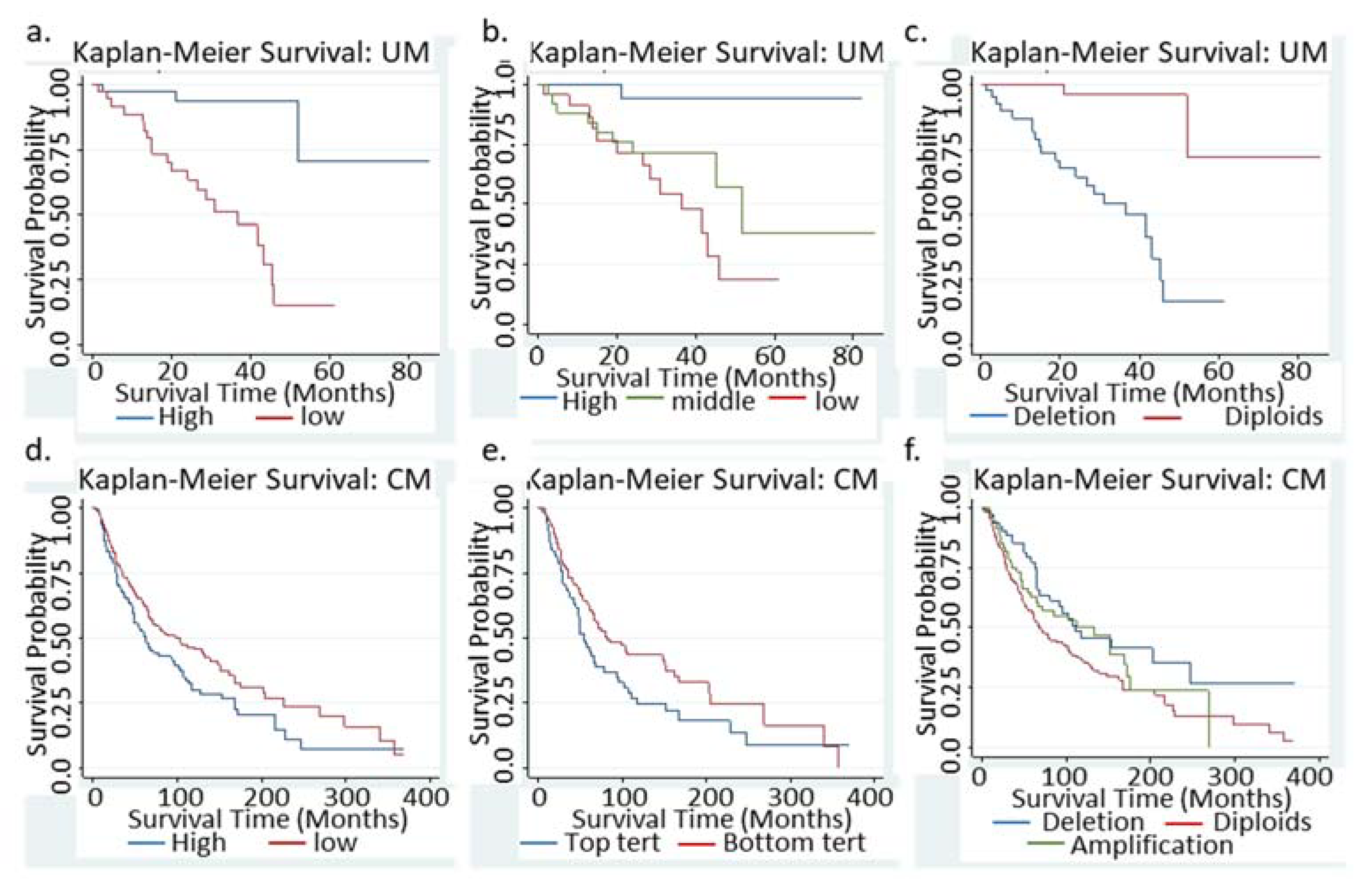
3.1. Low BAP1 mRNA or Loss of BAP1 Indicated Significant Worse Survival in UM Patients
3.2. Multivariate Cox Regression in UM Patients
3.3. Low BAP1 mRNA Indicated a Significant Better Survival in CM patients
3.4. Multivariate Cox Regression Analysis in CM Patients
3.5. Differential and Shared Molecular Networks of the BAP1 in CM and UM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdel-Rahman, M.H.; Pilarski, R.; Cebulla, C.M.; Massengill, J.B.; Christopher, B.N.; Boru, G.; Hovland, P.; Davidorf, F.H. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J. Med. Genet. 2011, 48, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Pilarski, R.; Cebulla, C.M.; Abdel-Rahman, M.H. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin. Genet. 2016, 89, 285–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirosawa, T.; Ishida, M.; Ishii, K.; Kanehara, K.; Kudo, K.; Ohnuma, S.; Kamei, T.; Motoi, F.; Naitoh, T.; Selaru, F.M.; et al. Loss of BAP1 expression is associated with genetic mutation and can predict outcomes in gallbladder cancer. PLoS ONE 2018, 13, e0206643. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Hebert, L.; Jacquemin, V.; Gad, S.; Caux-Moncoutier, V.; Dubois-d’Enghien, C.; Richaudeau, B.; Renaudin, X.; Sellers, J.; Nicolas, A.; et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am. J. Hum. Genet. 2013, 92, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, T.; Obenauf, A.C.; Murali, R.; Fried, I.; Griewank, K.G.; Ulz, P.; Windpassinger, C.; Wackernagel, W.; Loy, S.; Wolf, I.; et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 2011, 43, 1018–1021. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Seshasayee, D.; Noubade, R.; French, D.M.; Liu, J.; Chaurushiya, M.S.; Kirkpatrick, D.S.; Pham, V.C.; Lill, J.R.; Bakalarski, C.E.; et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012, 337, 1541–1546. [Google Scholar] [CrossRef] [Green Version]
- Pilarski, R.; Rai, K.; Cebulla, C.; Abdel-Rahman, M. BAP1 Tumor Predisposition Syndrome; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneReviews((R)): Seattle, WA, USA, 1993. [Google Scholar]
- Ewens, K.G.; Lalonde, E.; Richards-Yutz, J.; Shields, C.L.; Ganguly, A. Comparison of Germline versus Somatic BAP1 Mutations for Risk of Metastasis in Uveal Melanoma. BMC Cancer 2018, 18, 1172. [Google Scholar] [CrossRef]
- Decatur, C.L.; Ong, E.; Garg, N.; Anbunathan, H.; Bowcock, A.M.; Field, M.G.; Harbour, J.W. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol. 2016, 134, 728–733. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, S.J.; Robles-Espinoza, C.D.; McLellan, L.; Harrigan, J.; Jacq, X.; Hewinson, J.; Iyer, V.; Merchant, W.; Elliott, F.; Harland, M.; et al. A population-based analysis of germline BAP1 mutations in melanoma. Hum. Mol. Genet. 2017, 26, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ventii, K.H.; Devi, N.S.; Friedrich, K.L.; Chernova, T.A.; Tighiouart, M.; Van Meir, E.G.; Wilkinson, K.D. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008, 68, 6953–6962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H.; Sekido, Y.; et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef] [Green Version]
- Bononi, A.; Giorgi, C.; Patergnani, S.; Larson, D.; Verbruggen, K.; Tanji, M.; Pellegrini, L.; Signorato, V.; Olivetto, F.; Pastorino, S.; et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 2017, 546, 549–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matatall, K.A.; Agapova, O.A.; Onken, M.D.; Worley, L.A.; Bowcock, A.M.; Harbour, J.W. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer 2013, 13, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Taylor, M.; Miao, B.; Ji, Z.; Njauw, J.C.; Jönsson, G.; Frederick, D.T.; Tsao, H. BAP1 has a survival role in cutaneous melanoma. J. Investig. Dermatol. 2015, 135, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Van Essen, T.H.; van Pelt, S.I.; Versluis, M.; Bronkhorst, I.H.; Van Duinen, S.G.; Marinkovic, M.; Kroes, W.G.; Ruivenkamp, C.A.; Shukla, S.; De Klein, A.; et al. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br. J. Ophthalmol. 2014, 98, 1738–1743. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Wang, Z.; Huang, J.B.; Ren, X.D.; Ye, D.; Zhu, W.W.; Qin, L.X. Tissue-specific significance of BAP1 gene mutation in prognostic prediction and molecular taxonomy among different types of cancer. Tumour Biol. 2017, 39, 1010428317699111. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, S.M.; Miller, M.; Lee, P.; Leinonen, K.; Paquette, S.M.; Rodebaugh, Z.; Hahn, A.; Gibbs, D.L.; Slagel, J.; Longabaugh, W.J.; et al. The ISB Cancer Genomics Cloud: A Flexible Cloud-Based Platform for Cancer Genomics Research. Cancer Res. 2017, 77, e7–e10. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. Dewey, RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriello, G.; Cerami, E.; Aksoy, B.A.; Sander, C.; Schultz, N. Using MEMo to discover mutual exclusivity modules in cancer. Curr. Protoc. Bioinform. 2013, 41, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, F.Y.; Lavori, P.W. Lavori, Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin. Trials 2000, 21, 552–560. [Google Scholar] [CrossRef]
- Veitia, R.A.; Bottani, S.; Birchler, J.A. Gene dosage effects: Nonlinearities, genetic interactions, and dosage compensation. Trends Genet. 2013, 29, 385–393. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; Van Bodegom, A.; Paridaens, D.; Kiliç, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef]
- Masoomian, B.; Shields, J.A.; Shields, C.L. Overview of BAP1 cancer predisposition syndrome and the relationship to uveal melanoma. J. Curr. Ophthalmol. 2018, 30, 102–109. [Google Scholar] [CrossRef]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; De La Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Liu, F.; Bessonova, L.; Taylor, T.H.; Ziogas, A.; Meyskens, F.L., Jr.; Anton-Culver, H. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013, 26, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Liu-Smith, F.; Ziogas, A. An age-dependent interaction between sex and geographical UV index in melanoma risk. J. Am. Acad. Dermatol. 2017, 76. [Google Scholar] [CrossRef] [Green Version]
- Sime, W.; Niu, Q.; Abassi, Y.; Masoumi, K.C.; Zarrizi, R.; Køhler, J.B.; Kjellström, S.; Lasorsa, V.A.; Capasso, M.; Fu, H.; et al. BAP1 induces cell death via interaction with 14-3-3 in neuroblastoma. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]

| UM | CM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Variables | N | HR | 95% CI | p | N | HR | 95% CI | p | ||
| 50% Cutoff | High mRNA | 40 | ref | ref | ref | 235 | ref | ref | ref | ||
| Low mRNA | 40 | 9.57 | 2.82 | 32.48 | 0.010 | 234 | 0.73 | 0.56 | 0.95 | 0.019 | |
| mRNA in Tertiles | Highest | 26 | ref | ref | ref | ref | 158 | ref | ref | ref | |
| Middle | 28 | 8.27 | 1.04 | 65.56 | 0.045 | 155 | 0.73 | 0.53 | 1.02 | 0.063 | |
| Lowest | 26 | 14.46 | 1.89 | 110.64 | 0.010 | 156 | 0.67 | 0.48 | 0.93 | 0.017 | |
| BAP1-CNV | No alteration | 36 | ref | ref | ref | 227 | ref | ref | ref | ||
| Hemi. Dele. | 44 | 12.97 | 3.00 | 55.99 | 0.001 | 74 | 0.74 | 0.51 | 1.08 | 0.116 | |
| amplification | 0 | N/A | N/A | N/A | N/A | 66 | 0.56 | 0.37 | 0.84 | 0.005 | |
| UM | CM | Additional Variable(s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | HR | 95% CI | pBAP1 | p_av * | HR | 95% CI | pBAP1 | p_av * | |||
| 1 | 9.03 | 2.7 | 30.6 | <0.001 | 0.028 | 0.80 | 0.61 | 1.04 | 0.099 | <0.001 | Age (continuous) |
| 2 | 9.60 | 2.8 | 32.60 | <0.001 | 0.509 | 0.78 | 0.60 | 1.03 | 0.076 | <0.001 | Age as category ** |
| 3 | 8.85 | 2.6 | 30.5 | 0.001 | 0.565 | 0.70 | 0.53 | 0.94 | 0.019 | <0.001 | Stage *** |
| 4 | 9.59 | 2.3 | 32.5 | <0.001 | 0.299 | 0.73 | 0.56 | 0.95 | 0.019 | 0.396 | Sex |
| 5 | 0.71 | 0.52 | 0.99 | 0.042 | <0.001 | Ulceration | |||||
| 6 | 0.62 | 0.46 | 0.84 | 0.002 | <0.001 | Breslow Depth | |||||
| 7 | 10.13 | 2.96 | 34.63 | <0.0001 | 0.73 | 0.54 | 0.99 | 0.042 | Age, sex, stage | ||
| t-Test, p = 0.0004 | Cox Survival Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Age Group | N | Mean | Std. Err | HR | 95% CI | p_BAP1 | |
| ≤50 | 142 | 2640.1 | 75.7 | 1.00 | 0.59 | 1.68 | 0.995 |
| >50 | 326 | 3034.1 | 69.6 | 0.71 | 0.51 | 0.98 | 0.047 |
| All | 468 | 2914.5 | 85.1 | 0.73 | 0.56 | 0.95 | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu-Smith, F.; Lu, Y. Opposite Roles of BAP1 in Overall Survival of Uveal Melanoma and Cutaneous Melanoma. J. Clin. Med. 2020, 9, 411. https://doi.org/10.3390/jcm9020411
Liu-Smith F, Lu Y. Opposite Roles of BAP1 in Overall Survival of Uveal Melanoma and Cutaneous Melanoma. Journal of Clinical Medicine. 2020; 9(2):411. https://doi.org/10.3390/jcm9020411
Chicago/Turabian StyleLiu-Smith, Feng, and Yunxia Lu. 2020. "Opposite Roles of BAP1 in Overall Survival of Uveal Melanoma and Cutaneous Melanoma" Journal of Clinical Medicine 9, no. 2: 411. https://doi.org/10.3390/jcm9020411
APA StyleLiu-Smith, F., & Lu, Y. (2020). Opposite Roles of BAP1 in Overall Survival of Uveal Melanoma and Cutaneous Melanoma. Journal of Clinical Medicine, 9(2), 411. https://doi.org/10.3390/jcm9020411





