Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements at Baseline
2.3. Assessment of Physical Activity
2.4. Endpoints of the Study
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Post-Transplant Diabetes Mellitus
3.3. Cardiovascular and All-Cause Mortality
3.4. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ponton, P.; Rupolo, G.P. Quality-of-life change after kidney transplantation. Transplant. Proc. 2001, 33, 1887–1889. [Google Scholar] [CrossRef]
- Neale, J. Cardiovascular risk factors following renal transplant. World J. Transplant. 2015, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S. New-onset diabetes after transplantation—Should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients. Nephrol. Dial. Transplant. 2008, 23, 1816–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irons, B.K.; Mazzolini, T.A.; Greene, R.S. Delaying the Onset of Type 2 Diabetes Mellitus in Patients with Prediabetes. Pharmacotherapy 2004, 24, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Patrick, J. Skerrett. Physical activity and all-cause mortality: What is the dose-response relation? Med. Sci. Sports Exerc. 2001, 33, S459–S474. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Hernández-Sánchez, S.; Garofalo, C.; Ruiz, J.R.; Carrero, J.J.; Bellizzi, V. Exercise training in kidney transplant recipients: A systematic review. J. Nephrol. 2019, 32, 567–579. [Google Scholar] [CrossRef]
- Zelle, D.M.; Klaassen, G.; Van Adrichem, E.; Bakker, S.J.L.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152. [Google Scholar] [CrossRef]
- Macdonald, J.H.; Kirkman, D.; Jibani, M. Kidney Transplantation: A Systematic Review of Interventional and Observational Studies of Physical Activity on Intermediate Outcomes. Adv. Chronic Kidney Dis. 2009, 16, 482–500. [Google Scholar] [CrossRef]
- Bellizzi, V.; Cupisti, A.; Capitanini, A.; Calella, P.; D’Alessandro, C. Physical activity and renal transplantation. Kidney Blood Press. Res. 2014, 39, 212–219. [Google Scholar] [CrossRef]
- Takahashi, A.; Hu, S.L.; Bostom, A. Physical Activity in Kidney Transplant Recipients: A Review. Am. J. Kidney Dis. 2018, 72, 433–443. [Google Scholar] [CrossRef]
- Kang, A.W.; Garber, C.E.; Eaton, C.B.; Risica, P.M. Physical Activity and Cardiovascular Risk among Kidney Transplant Patients. Med. Sci. Sport. Exerc. 2019, 51, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Kok, T.; Dontje, M.L.; Danchell, E.I.; Navis, G.; Van Son, W.J.; Bakker, S.J.L.; Corpeleijn, E. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin. Transplant. 2013, 27, E484–E490. [Google Scholar] [CrossRef] [PubMed]
- Orazio, L.; Hickman, I.; Armstrong, K.; Johnson, D.; Banks, M.; Isbel, N. Higher Levels of Physical Activity Are Associated With a Lower Risk of Abnormal Glucose Tolerance in Renal Transplant Recipients. J. Ren. Nutr. 2009, 19, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Corpeleijn, E.; Stolk, R.P.; de Greef, M.H.G.; Gans, R.O.B.; van der Heide, J.J.H.; Navis, G.; Bakker, S.J.L. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 898–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Janaudis-Ferreira, T.; Mathur, S.; Deliva, R.; Howes, N.; Patterson, C.; Räkel, A.; So, S.; Wickerson, L.; White, M.; Avitzur, Y.; et al. Exercise for Solid Organ Transplant Candidates and Recipients: A Joint Position Statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation 2019, 103, e220–e238. [Google Scholar] [CrossRef]
- Dontje, M.L.; de Greef, M.H.G.; Krijnen, W.P.; Corpeleijn, E.; Kok, T.; Bakker, S.J.L.; Stolk, R.P.; van der Schans, C.P. Longitudinal measurement of physical activity following kidney transplantation. Clin. Transplant. 2014, 28, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Nielens, H.; Lejeune, T.M.; Lalaoui, A.; Squifflet, J.P.; Pirson, Y.; Goffin, E. Increase of physical activity level after successful renal transplantation: A 5 year follow-up study. Nephrol. Dial. Transplant. 2001, 16, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Conti, D.; Siminoff, L.A. Prevalence and determinants of physical activity and fluid intake in kidney transplant recipients. Clin. Transplant. 2010, 24, E69–E81. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.; Labriola, M.; Christensen, K.B.; Bültmann, U.; Villadsen, E. Physical work environment risk factors for long term sickness absence: Prospective findings among a cohort of 5357 employees in Denmark. Br. Med. J. 2006, 332, 449–451. [Google Scholar] [CrossRef] [Green Version]
- Huai, P.; Xun, H.; Reilly, K.H.; Wang, Y.; Ma, W.; Xi, B. Physical activity and risk of hypertension a meta-analysis of prospective cohort studies. Hypertension 2013, 62, 1021–1026. [Google Scholar] [CrossRef]
- Larsson, C.A.; Krøll, L.; Bennet, L.; Gullberg, B.; Råstam, L.; Lindblad, U. Leisure time and occupational physical activity in relation to obesity and insulin resistance: A population-based study from the Skaraborg Project in Sweden. Metabolism. 2012, 61, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Engberink, M.F.; Brink, E.J.; Van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Joosten, M.M.; Gans, R.O.B.; Navis, G.; et al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1811–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Berg, E.; Engberink, M.F.; Brink, E.J.; Van Baak, M.A.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Dietary protein, blood pressure and renal function in renal transplant recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, E.; Johanna, M.; Geleijnse, E.; Brink, J.; van Baak, M.A.; van der Heide, J.J.H.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 3352–3359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Kramer, H.J.; Griffin, K.A.; Vellanki, K.; Leehey, D.J.; Bansal, V.K.; Markossian, T.W.; Levey, A.; Stevens, L.; Schmid, C.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; Gansevoort, R.T.; Schouten, J.P.; de Jong, P.E.; Gans, R.O.B.; Bakker, S.J.L. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 2009, 207, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Byambasukh, O.; Snieder, H.; Corpeleijn, E. The relation between leisure time, commuting and occupational physical activity with blood pressure in 125,402 adults: The Lifelines cohort. J. Am. Heart Assoc. 2019, 8, e0814313. [Google Scholar]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernandez, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onser diabetes after transplantation: 2003 International Consensus Guidelines. Transplantation 2003, 75, SS3–SS24. [Google Scholar] [CrossRef]
- Sharif, A.; Hecking, M.; De Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osté, M.C.J.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; Van Den Berg, E.; Postmus, D.; De Borst, M.H.; Kromhout, D.; Bakker, S.J.L. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaderna, H.; Zahn, D.; Pretsch, J.; Connor, S.L.; Zittermann, A.; Schulze Schleithoff, S.; Bramstedt, K.A.; Smits, J.M.A.; Weidner, G. Dietary habits are related to outcomes in patients with advanced heart failure awaiting heart transplantation. J. Card. Fail. 2013, 19, 240–250. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Shah, S. Prevention of Cardiovascular Disease: Guideline for Assessment and Management of Cardiovascular Risk; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Weir, M.R.; Fink, J.C. Risk for posttransplant diabetes mellitus with current immunosuppressive medications. Am. J. Kidney Dis. 1999, 34, 1–13. [Google Scholar] [CrossRef]
- Sharif, A.; Moore, R.; Baboolal, K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation 2008, 85, 353–358. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Morrato, E.H.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care 2005, 28, 1599. [Google Scholar] [CrossRef] [Green Version]
- Robinson-Cohen, C.; Katz, R.; Mozaffarian, D.; Dalrymple, L.S.; De Boer, I.; Sarnak, M.; Shlipak, M.; Siscovick, D.; Kestenbaum, B. Physical activity and rapid decline in kidney function among older adults. Arch. Intern. Med. 2009, 169, 2116–2123. [Google Scholar] [CrossRef] [Green Version]
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Weiss, N.S.; Sachs, M.C.; Ruzinski, J.; Kundzins, J.; Rock, D.; de Boer, I.H.; Ikizler, T.A.; et al. Physical Activity and Change in Estimated GFR among Persons with CKD. J. Am. Soc. Nephrol. 2014, 25, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Yucel, R.; Conti, D.; Siminoff, L.A. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transpl. Int. 2009, 22, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, V.A.; Yki-Järvinen, H.; DeFronzo, R.A. Physical training and insulin sensitivity. Diabetes. Metab. Rev. 1986, 1, 445–481. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.D.; Stefanick, M.L.; Williams, P.T.; Haskell, W.L. The Effects on Plasma Lipoproteins of a Prudent Weight-Reducing Diet, With or Without Exercise, in Overweight Men and Women. N. Engl. J. Med. 1991, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.B.; de Borst, M.H.; van den Berg, E.; Soedamah-Muthu, S.S.; Kromhout, D.; Navis, G.J.; Bakker, S.J.L. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am. J. Transplant. 2018, 10, 2523–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byambasukh, O.; Zelle, D.; Corpeleijn, E. Physical Activity, Fatty Liver, and Glucose Metabolism Over the Life Course. Am. J. Gastroenterol. 2019, 114, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Crandall, J.; Schade, D.; Ma, Y.; Fujimoto, W.Y.; Barrett-Connor, E.; Fowler, S.; Dagogo-Jack, S.; Andres, R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1075–1081. [Google Scholar]
- Wagenmakers, R.; Van Den Akker-Scheek, I.; Groothoff, J.W.; Zijlstra, W.; Bulstra, S.K.; Kootstra, J.W.J.; Wendel-Vos, G.C.W.; Van Raaij, J.J.A.M.; Stevens, M. Reliability and validity of the short questionnaire to assess health-enhancing physical activity (SQUASH) in patients after total hip arthroplasty. BMC Musculoskelet. Disord. 2008, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Arends, S.; Hofman, M.; Kamsma, Y.P.T.; van der Veer, E.; Houtman, P.M.; Kallenberg, C.G.M.; Spoorenberg, A.; Brouwer, E. Daily physical activity in ankylosing spondylitis: Validity and reliability of the IPAQ and SQUASH and the relation with clinical assessments. Arthritis Res. Ther. 2013, 15, R99. [Google Scholar] [CrossRef] [Green Version]
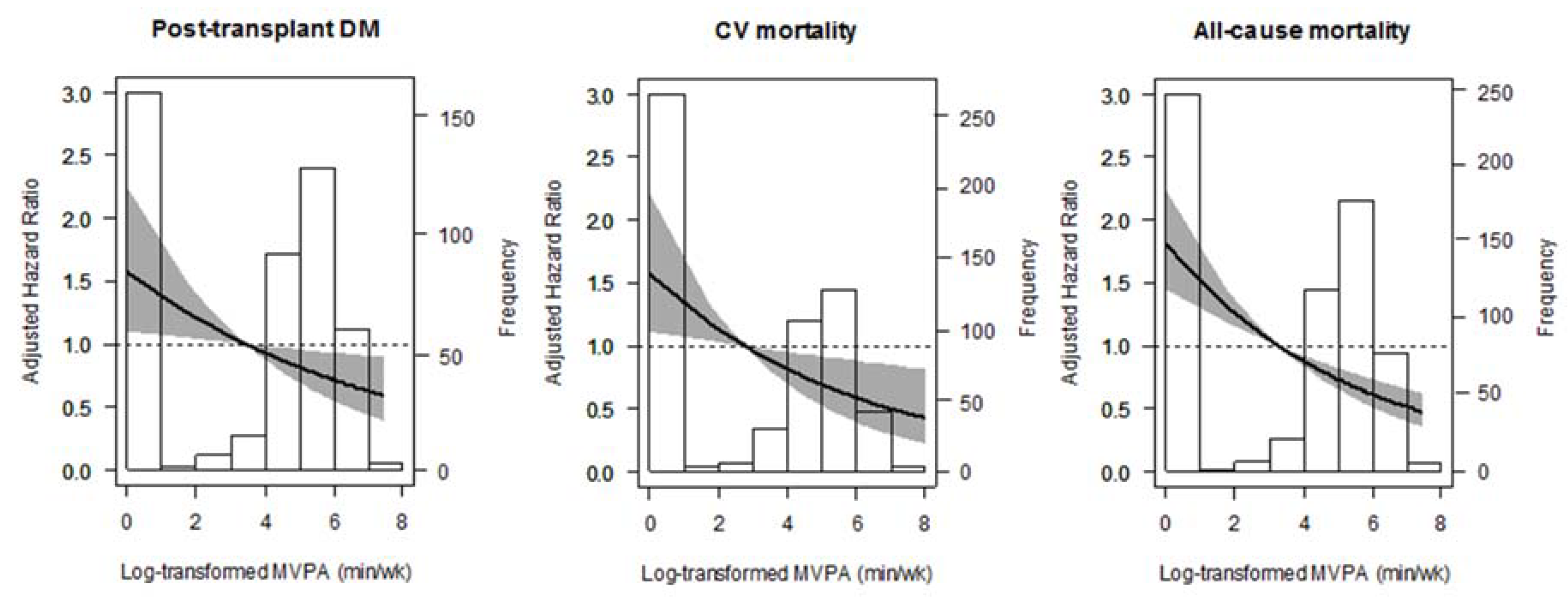

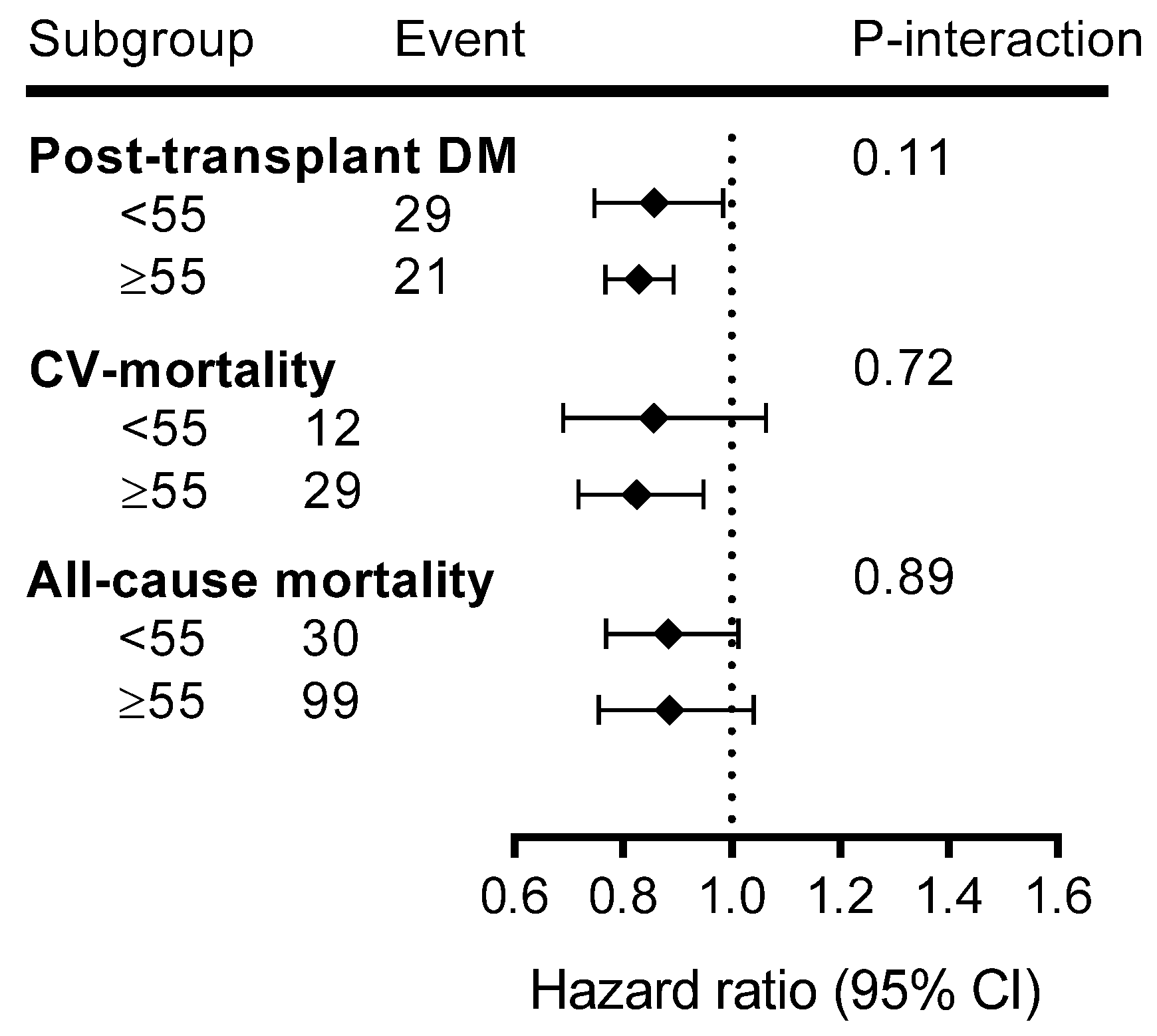
| Variable | Total (n = 650) | No-MVPA (n = 246) | MVPA-1 (n = 201) | MVPA-2 (n = 203) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 52.6 ± 12.8 | 54.1 ± 11.8 | 51.9 ± 13.4 | 51.6 ± 13.3 | 0.08 |
| Male gender (%, n) | 56.3 (366) | 56.1 (138) | 55.7 (112) | 57.1 (116) | 0.96 |
| Current smoking (%, n) | 12.3 (80) | 15.3 (36) | 12.4 (25) | 9.5 (19) | 0.20 |
| Occupational status: Employed * (%, n) | 49.5 (322) | 44.7 (110) | 51.2 (103) | 53.7 (109) | 0.14 |
| Alcohol use (g/day) | 2.61 (0.1–11.1) | 1.45 (0.1–9.7) | 2.51 (0.1–9.7) | 3.95 (0.1–14.1) | 0.01 |
| Total energy intake (kcal/d) | 2174.9 ± 640.7 | 2114.7± 720.4 | 2247.5 ± 598.7 | 2173.2 ± 573.9 | 0.11 |
| Non-occupational MVPA (min/week) | 90 (0–240) | 0 | 120 (60–150) | 360 (260–540) | − |
| Anthropometric measures | |||||
| Body mass index (kg/m2) | 26.7 ± 4.84 | 27.9 ± 5.49 | 25.7 ± 4.26 | 26.1 ± 4.23 | 0.001 |
| Waist circumference, men (cm) | 101.1 ± 13.4 | 104.0 ± 13.7 | 98.9 ± 13.5 | 99.7 ± 12.4 | 0.01 |
| Waist circumference, women (cm) | 95.0 ± 15.8 | 99.7 ± 12.4 | 91.5 ± 14.6 | 93.1 ± 14.1 | 0.01 |
| Creatinine excretion (mmol/24h) | 11.7 ± 3.49 | 11.2 ± 3.80 | 11.8 ± 3.29 | 12.2 ± 3.23 | 0.01 |
| Lipids and blood pressure | |||||
| Total cholesterol (mmol/L) | 5.14 ± 1.11 | 5.18 ± 1.18 | 5.17 ± 1.10 | 5.07 ± 1.02 | 0.48 |
| Triglyceride (mmol/L) | 1.68 (1.2–2.33) | 1.85 (1.3–2.6) | 1.67 (1.2–2.4) | 1.59 (1.2–2.05) | 0.001 |
| HDL-C in men (mmol/L) | 1.27 ± 0.41 | 1.23 ± 0.41 | 1.27 ± 0.36 | 1.32 ± 0.45 | 0.21 |
| HDL-C in women (mmol/L) | 1.56 ± 0.51 | 1.39 ± 0.43 | 1.62 ± 0.54 | 1.70 ± 0.53 | 0.001 |
| Systolic blood pressure (mm Hg) | 136.2 ± 17.3 | 138.3 ± 18.5 | 135.6 ± 16.7 | 134.2 ± 16.3 | 0.04 |
| Diastolic blood pressure (mm Hg) | 82.8 ± 10.9 | 83.1 ± 11.2 | 82.5 ± 11.3 | 82.6 ± 10.3 | 0.77 |
| Cardiovascular medication use | |||||
| Antihypertensive (%, n) | 88 (572) | 92.7 (228) | 81.1 (163) | 89.2 (181) | 0.001 |
| A2 antagonist (%, n) | 14.8 (96) | 15.4 (38) | 12.9 (26) | 15.8 (32) | 0.68 |
| ACE inhibitor (%, n) | 32.2 (209) | 32.1 (79) | 30.3 (61) | 34.0 (69) | 0.74 |
| RAAS blockers (%, n) | 47.8 (311) | 49.2 (121) | 44.8 (90) | 49.3 (100) | 0.58 |
| Beta-blockers (%, n) | 63.2 (411) | 63.4 (156) | 62.2 (125) | 64.0 (130) | 0.93 |
| Calcium channel blockers (%, n) | 24.5 (159) | 26.0 (64) | 20.4 (41) | 26.6 (54) | 0.27 |
| Diuretics (%, n) | 40.0 (260) | 52.0 (128) | 27.4 (50) | 37.9 (77) | 0.001 |
| Vitamin K antagonist (%, n) | 11.4 (74) | 13 (32) | 10.4 (21) | 10.3 (21) | 0.60 |
| mTOR inhibitor (%, n) | 1.8 (12) | 3.3 (8) | 1 (2) | 1 (2) | 0.60 |
| Anti-diabetic drugs (%, n) | 14.8 (96) | 18.7 (46) | 14.4 (29) | 10.3 (21) | 0.045 |
| Statin (%, n) | 51.8 (337) | 54.9 (135) | 52.7 (106) | 47.3 (96) | 0.27 |
| Glucose metabolism | |||||
| Fasting plasma glucose (mmol/L) | 5.67 ± 1.82 | 5.78 ± 192 | 5.76 ± 2.13 | 5.46 ± 1.28 | 0.13 |
| Heamoglobin A1C (%) | 5.94 ± 0.78 | 6.03 ± 0.77 | 5.94 ± 0.90 | 5.83 ± 0.65 | 0.021 |
| Kidney function | |||||
| eGFR (mL/min/1.73m2) | 52.0 ± 20.2 | 49.9 ± 22.1 | 53.8 ± 18.7 | 52.9 ± 18.8 | 0.09 |
| Albumin excretion (mg/24h) | 267.3 ± 734.6 | 307.2 ± 777.5 | 175.1 ± 378.5 | 308.7 ± 917.5 | 0.11 |
| Proteinuria (%, n) | 21.5 (140) | 28.0 (69) | 16.9 (34) | 18.2 (37) | 0.01 |
| Primary renal disease (%, n) | 0.01 | ||||
| Glomerulosclerosis | 28.8 (187) | 30.1 (74) | 28.4 (57) | 27.6 (56) | |
| Glomerulonephritis | 7.7 (50) | 5.7 (14) | 8.0 (16) | 9.9 (20) | |
| Tubulointerstitial nephritis | 11.8 (77) | 9.8 (24) | 12.9 (26) | 13.3 (27) | |
| Polycystic kidney disease | 20.9 (136) | 20.7 (51) | 19.9 (40) | 22.2 (45) | |
| Renal hypodysplasia | 3.5 (23) | 4.1 (10) | 3.0 (6) | 3.4 (7) | |
| Renavascular diseases | 5.7 (37) | 7.7 (19) | 4.0 (8) | 4.9 (10) | |
| Diabetes mellitus | 4.6 (30) | 6.5 (16) | 5.5 (11) | 1.5 (3) | |
| Others | 16.9 (110) | 15.4 (38) | 18.4 (37) | 17.2 (35) | |
| Duration of dialysis before the transplantation (months) | 25 (8–48) | 29 (11–51) | 19 (4–49) | 25 (9–43) | 0.51 |
| Transplant characteristics | |||||
| Transplant vintage (months) | 14.0 (2.0–39.5) | 17.0 (2.0–41.0) | 12.0 (2.0–44.8) | 16.0 (0.5–41.0) | 0.49 |
| Cold ischemia time (h) | 15.2 (2.8–21.1) | 16.4 (3.6–22.0) | 15.1 (2.6–21.3) | 13.6 (2.5–20.5) | 0.10 |
| Living donor (%, n) | 34.8 (226) | 26.4 (65) | 37.3 (75) | 42.4 (86) | 0.001 |
| Pre-emptive transplant (%, n) | 16.6 (108) | 13.4 (33) | 20.9 (42) | 16.3 (33) | 0.11 |
| Acute rejection | 27.2 (177) | 27.2 (67) | 27.9 (56) | 26.6 (54) | 0.96 |
| Immunosuppressive medication | |||||
| Calcineurin inhibitor (%, n) | 58.3 (379) | 59.8 (147) | 59.2 (119) | 55.7 (113) | 0.52 |
| Proliferation inhibitor (%, n) | 82.6 (537) | 80.1 (197) | 84.1 (169) | 84.2 (171) | 0.62 |
| Prednisolone dose (mg) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 0.48 |
| Physical Activity | MVPA (cont.) | No-MVPA (Ref) | MVPA-1 | MVPA-2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Post-transplant DM | |||||||
| No. of events | 50/502 | 23 | 14 | 13 | |||
| Model 1 | 0.88 (0.79–0.97) | 0.01 | 1.00 | 0.57 (0.29–1.10) | 0.09 | 0.49 (0.25–0.96) | 0.04 |
| Model 2 | 0.88 (0.79–0.98) | 0.02 | 1.00 | 0.61 (0.31–1.20) | 0.15 | 0.49 (0.25–0.96) | 0.04 |
| Model 3 | 0.88 (0.79–0.98) | 0.02 | 1.00 | 0.55 (0.28–1.07) | 0.08 | 0.48 (0.24–0.95) | 0.04 |
| Model 4 | 0.87 (0.79–0.97) | 0.01 | 1.00 | 0.57 (0.29–1.12) | 0.10 | 0.46 (0.22–0.94) | 0.03 |
| Model 5 | 0.89 (0.80–0.99) | 0.03 | 1.00 | 0.59 (0.30–1.26) | 0.11 | 0.52 (0.26–1.03) | 0.06 |
| Model 6 | 0.91 (0.82–1.01) | 0.09 | 1.00 | 0.70 (0.36–1.40) | 0.31 | 0.60 (0.29–1.22) | 0.16 |
| Model 7 | 0.88 (0.79–0.99) | 0.03 | 1.00 | 0.63 (0.31–1.25) | 0.19 | 0.50 (0.25–1.03) | 0.06 |
| Model 8 | 0.87 (0.79–0.97) | 0.01 | 1.00 | 0.55 (0.29–1.08) | 0.08 | 0.47 (0.24–0.93) | 0.03 |
| Model 9 | 0.91 (0.82–1.01) | 0.12 | 1.00 | 0.72 (0.36–1.41) | 0.34 | 0.59 (0.30–1.19) | 0.14 |
| Model 10 | 0.87 (0.78–0.97) | 0.01 | 1.00 | 0.58 (0.32–1.25) | 0.11 | 0.44 (0.21–0.92) | 0.03 |
| Cardiovascular mortality | |||||||
| No. of events | 53/589 | 26 | 14 | 13 | |||
| Model 1 | 0.84 (0.74–0.94) | 0.01 | 1.00 | 0.45 (0.22–0.94) | 0.03 | 0.34 (0.15–0.77) | 0.01 |
| Model 2 | 0.84 (0.75–0.95) | 0.01 | 1.00 | 0.49 (0.23–1.02) | 0.06 | 0.35 (0.16–0.80) | 0.01 |
| Model 3 | 0.86 (0.76–0.96) | 0.01 | 1.00 | 0.51 (0.25–1.05) | 0.07 | 0.40 (0.18–0.91) | 0.03 |
| Model 4 | 0.87 (0.77–0.98) | 0.02 | 1.00 | 0.56 (0.26–1.21) | 0.14 | 0.43 (0.19–0.94) | 0.046 |
| Model 5 | 0.84 (0.74–0.94) | 0.001 | 1.00 | 0.45 (0.21–0.93) | 0.03 | 0.36 (0.16–0.81) | 0.01 |
| Model 6 | 0.85 (0.76–0.96) | 0.001 | 1.00 | 0.49 (0.23–1.02) | 0.06 | 0.38 (0.17–0.86) | 0.02 |
| Model 7 | 0.85 (0.75–0.96) | 0.01 | 1.00 | 0.51 (0.23–1.11) | 0.09 | 0.40 (0.17–0.92) | 0.03 |
| Model 8 | 0.87 (0.77–0.98) | 0.02 | 1.00 | 0.55 (0.26–1.16) | 0.12 | 0.44 (0.19–0.99) | 0.051 |
| All-cause mortality | |||||||
| No. of events | 129/650 | 76 | 27 | 26 | |||
| Model 1 | 0.84 (0.78–0.89) | <0.001 | 1.00 | 0.39 (0.25–0.61) | <0.001 | 0.37 (0.24–0.58) | <0.001 |
| Model 2 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.43 (0.27–0.67) | <0.001 | 0.40 (0.26–0.63) | <0.001 |
| Model 3 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.41 (0.27–0.64) | <0.001 | 0.41 (0.26–0.64) | <0.001 |
| Model 4 | 0.83 (0.77–0.89) | <0.001 | 1.00 | 0.41 (0.21–0.64) | <0.001 | 0.35 (0.22–0.58) | <0.001 |
| Model 5 | 0.83 (0.78–0.89) | <0.001 | 1.00 | 0.39 (0.25–0.61) | <0.001 | 0.37 (0.23–0.58) | <0.001 |
| Model 6 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.42 (0.27–0.66) | <0.001 | 0.41 (0.26–0.65) | <0.001 |
| Model 7 | 0.84 (0.78–0.89) | <0.001 | 1.00 | 0.40 (0.25–0.63) | <0.001 | 0.37 (0.23–0.59) | <0.001 |
| Model 8 | 0.86 (0.80–0.92) | <0.001 | 1.00 | 0.45 (0.29–0.70) | <0.001 | 0.44 (0.28–0.69) | <0.001 |
| Physical Activity | MVPA (cont.) | No-MVPA | MVPA > 0 | ||||
|---|---|---|---|---|---|---|---|
| HR^ (95% CI) | p-value | N * | Reference | N * | HR^^ (95% CI) | p-Value | |
| Post-transplant DM | |||||||
| Non-occupational PA | 0.87 (0.74–1.03) | 0.113 | 10 | 1.00 | 10 | 0.46 (0.18–1.13) | 0.076 |
| Total PA | 0.91 (0.78–1.06) | 0.212 | 8 | 1.00 | 12 | 0.48 (0.20–1.20) | 0.056 |
| Cardiovascular mortality | |||||||
| Non-occupational PA | 0.63 (0.48–0.83) | 0.001 | 12 | 1.00 | 3 | 0.11 (0.11–0.42) | 0.001 |
| Total PA | 0.75 (0.63–0.91) | 0.003 | 9 | 1.00 | 6 | 0.23 (0.10–0.58) | 0.051 |
| All-cause mortality | |||||||
| Non-occupational PA | 0.76 (0.66–0.87) | <0.01 | 25 | 1.00 | 11 | 0.21 (0.14–0.51) | <0.01 |
| Total PA | 0.82 (0.74–0.92) | 0.001 | 19 | 1.00 | 17 | 0.30 (0.18–0.58) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambasukh, O.; Osté, M.C.J.; Gomes-Neto, A.W.; van den Berg, E.; Navis, G.; Bakker, S.J.L.; Corpeleijn, E. Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 415. https://doi.org/10.3390/jcm9020415
Byambasukh O, Osté MCJ, Gomes-Neto AW, van den Berg E, Navis G, Bakker SJL, Corpeleijn E. Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. Journal of Clinical Medicine. 2020; 9(2):415. https://doi.org/10.3390/jcm9020415
Chicago/Turabian StyleByambasukh, Oyuntugs, Maryse C. J. Osté, António W. Gomes-Neto, Else van den Berg, Gerjan Navis, Stephan J. L. Bakker, and Eva Corpeleijn. 2020. "Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients" Journal of Clinical Medicine 9, no. 2: 415. https://doi.org/10.3390/jcm9020415
APA StyleByambasukh, O., Osté, M. C. J., Gomes-Neto, A. W., van den Berg, E., Navis, G., Bakker, S. J. L., & Corpeleijn, E. (2020). Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. Journal of Clinical Medicine, 9(2), 415. https://doi.org/10.3390/jcm9020415





