Thromboembolic Events Are Independently Associated with Liver Stiffness in Patients with Fontan Circulation
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Clinical Data
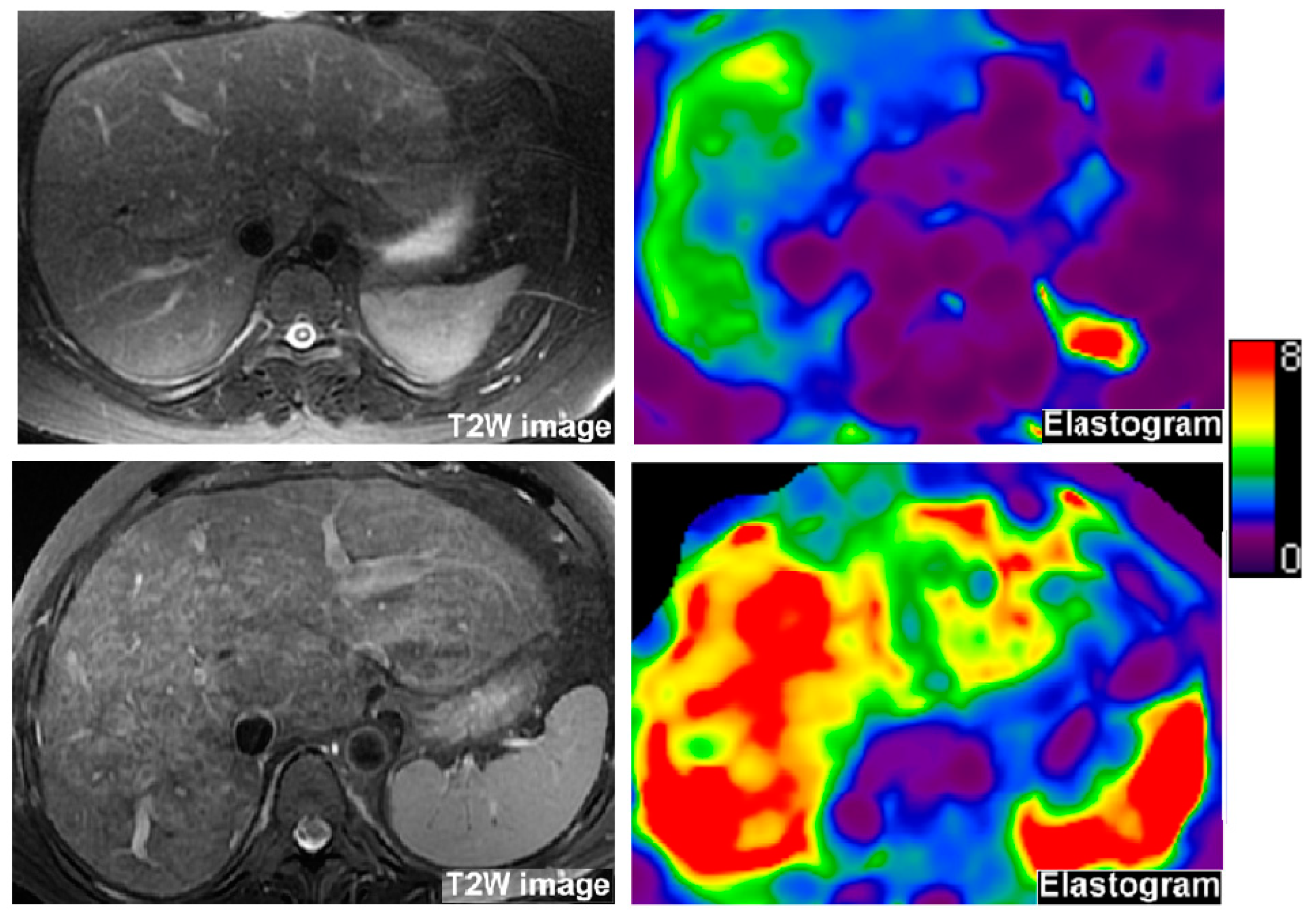
2.3. Liver MRE
2.4. Ultrasound SWE
2.5. Cardiac MRI
2.6. Cardiac Catheterization
2.7. Cardiopulmonary Exercise Testing
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- de Leval, M.R.; Kilner, P.; Gewillig, M.; Bull, C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef]
- Gewillig, M. The Fontan circulation. Heart 2005, 91, 839–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, C.J.; Bradley, E.A.; Landzberg, M.J.; Aboulhosn, J.; Beekman, R.H., 3rd; Book, W.; Gurvitz, M.; John, A.; John, B.; Marelli, A.; et al. Fontan-Associated Liver Disease: Proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J. Am. Coll. Cardiol. 2017, 70, 3173–3194. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Bokma, J.P.; Engel, M.E.; Kuijpers, J.M.; Hanke, S.P.; Zuhlke, L.; Zhang, B.; Veldtman, G.R. Factors associated with long-term mortality after Fontan procedures: A systematic review. Heart 2017, 103, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, C.H.; Sheron, N.; Vettukattill, J.J.; Hacking, N.; Stedman, B.; Millward-Sadler, H.; Haw, M.; Cope, R.; Salmon, A.P.; Sivaprakasam, M.C.; et al. Hepatic changes in the failing Fontan circulation. Heart 2007, 93, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychik, J.; Veldtman, G.; Rand, E.; Russo, P.; Rome, J.J.; Krok, K.; Goldberg, D.J.; Cahill, A.M.; Wells, R.G. The precarious state of the liver after a Fontan operation: Summary of a multidisciplinary symposium. Pediatr. Cardiol. 2012, 33, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, D.J.; Surrey, L.F.; Glatz, A.C.; Dodds, K.; O’Byrne, M.L.; Lin, H.C.; Fogel, M.; Rome, J.J.; Rand, E.B.; Russo, P.; et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Egbe, A.; Miranda, W.R.; Connolly, H.M.; Khan, A.R.; Al-Otaibi, M.; Venkatesh, S.K.; Simonetto, D.; Kamath, P.; Warnes, C. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. Int. J. Cardiol. 2018, 258, 299–304. [Google Scholar] [CrossRef]
- Diaz, E.S.; Dillman, J.R.; Veldtman, G.R.; Trout, A.T. MRI measured liver stiffness does not predict focal liver lesions after the Fontan operation. Pediatr. Radiol. 2018. [Google Scholar] [CrossRef]
- Alsaied, T.; Alsidawi, S.; Allen, C.C.; Faircloth, J.; Palumbo, J.S.; Veldtman, G.R. Strategies for thromboprophylaxis in Fontan circulation: A meta-analysis. Heart 2015, 101, 1731–1737. [Google Scholar] [CrossRef]
- Georgekutty, J.; Kazerouninia, A.; Wang, Y.; Ermis, P.R.; Parekh, D.R.; Franklin, W.J.; Lam, W.W. Novel oral anticoagulant use in adult Fontan patients: A single center experience. Congenit. Heart Dis. 2018, 13, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Sadiq, F.; Anstee, Q.M.; Levene, A.P.; Goldin, R.D.; Thursz, M.R. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Goldin, R.; Hellier, S.; Knapp, S.; Frodsham, A.; Hennig, B.; Hill, A.; Apple, R.; Cheng, S.; Thomas, H.; et al. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut 2003, 52, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jang, Y.N.; Song, J.S. Comparison of gradient-recalled echo and spin-echo echo-planar imaging MR elastography in staging liver fibrosis: A meta-analysis. Eur. Radiol. 2018, 28, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Serai, S.D.; Dillman, J.R.; Trout, A.T. Spin-echo Echo-planar Imaging MR Elastography versus Gradient-echo MR Elastography for Assessment of Liver Stiffness in Children and Young Adults Suspected of Having Liver Disease. Radiology 2017, 282, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019. [Google Scholar] [CrossRef]
- Northern, N.A.; Dillman, J.R.; Trout, A.T. Frequency of technical success of two-dimensional ultrasound shear wave elastography in a large pediatric and young adult cohort: A clinical effectiveness study. Pediatr. Radiol. 2019. [Google Scholar] [CrossRef]
- Bossers, S.S.; Helbing, W.A.; Duppen, N.; Kuipers, I.M.; Schokking, M.; Hazekamp, M.G.; Bogers, A.J.; Ten Harkel, A.D.; Takken, T. Exercise capacity in children after total cavopulmonary connection: Lateral tunnel versus extracardiac conduit technique. J. Thorac. Cardiovasc. Surg. 2014, 148, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [Green Version]
- Arena, R.; Myers, J.; Abella, J.; Pinkstaff, S.; Brubaker, P.; Moore, B.; Kitzman, D.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; et al. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ. Heart Fail. 2009, 2, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Alsaied, T.; Sleeper, L.A.; Masci, M.; Ghelani, S.J.; Azcue, N.; Geva, T.; Powell, A.J.; Rathod, R.H. Maldistribution of pulmonary blood flow in patients after the Fontan operation is associated with worse exercise capacity. J. Cardiovasc. Magnet. Reson. 2018, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P.; Cochrane, A.; McCrindle, B.; Benson, L.; Williams, W.; Andrew, M. Thromboembolic complications after fontan procedures--the role of prophylactic anticoagulation. J. Thorac. Cardiovascul. Surg. 1998, 115, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Khairy, P.; Fernandes, S.M.; Mayer, J.E., Jr.; Triedman, J.K.; Walsh, E.P.; Lock, J.E.; Landzberg, M.J. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008, 117, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monagle, P.; Cochrane, A.; Roberts, R.; Manlhiot, C.; Weintraub, R.; Szechtman, B.; Hughes, M.; Andrew, M.; McCrindle, B.W.; Fontan Anticoagulation Study, G. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J. Am. Coll. Cardiol. 2011, 58, 645–651. [Google Scholar] [CrossRef] [Green Version]
- McCrindle, B.W.; Manlhiot, C.; Cochrane, A.; Roberts, R.; Hughes, M.; Szechtman, B.; Weintraub, R.; Andrew, M.; Monagle, P.; Fontan Anticoagulation Study, G. Factors associated with thrombotic complications after the Fontan procedure: A secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J. Am. Coll. Cardiol. 2013, 61, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Jahangiri, M.; Shore, D.; Kakkar, V.; Lincoln, C.; Shinebourne, E. Coagulation factor abnormalities after the Fontan procedure and its modifications. J. Thorac. Cardiovasc. Surg. 1997, 113, 989–992, discussion 992–983. [Google Scholar] [CrossRef] [Green Version]
- Marrone, C.; Galasso, G.; Piccolo, R.; de Leva, F.; Paladini, R.; Piscione, F.; Santoro, G. Antiplatelet versus anticoagulation therapy after extracardiac conduit Fontan: A systematic review and meta-analysis. Pediatr. Cardiol. 2011, 32, 32–39. [Google Scholar] [CrossRef]
- Binotto, M.A.; Maeda, N.Y.; Lopes, A.A. Altered endothelial function following the Fontan procedure. Cardiol. Young 2008, 18, 70–74. [Google Scholar] [CrossRef]
- Binotto, M.A.; Maeda, N.Y.; Lopes, A.A. Evidence of endothelial dysfunction in patients with functionally univentricular physiology before completion of the Fontan operation. Cardiol. Young 2005, 15, 26–30. [Google Scholar] [CrossRef]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.M.; Opotowsky, A.R.; Raza, R.; Harney, S.; Ukomadu, C.; Landzberg, M.J.; Valente, A.M.; Breitbart, R.E.; Singh, M.N.; Gauvreau, K.; et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit. Heart Dis. 2014, 9, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Dillman, J.R.; Xanthakos, S.; Kohli, R.; Sprague, G.; Serai, S.; Mahley, A.D.; Podberesky, D.J. Prospective Assessment of Correlation between US Acoustic Radiation Force Impulse and MR Elastography in a Pediatric Population: Dispersion of US Shear-Wave Speed Measurement Matters. Radiology 2016, 281, 544–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talwalkar, J.A.; Kurtz, D.M.; Schoenleber, S.J.; West, C.P.; Montori, V.M. Ultrasound-based transient elastography for the detection of hepatic fibrosis: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2007, 5, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sepulveda, J.A.; Fonseca, Y.; Vodkin, I.; Vaughn, G.; Newbury, R.; Vavinskaya, V.; Dwek, J.; Perry, J.C.; Reshamwala, P.; Baehling, C.; et al. Evaluation of Fontan liver disease: Correlation of transjugular liver biopsy with magnetic resonance and hemodynamics. Congenit. Heart Dis. 2019. [Google Scholar] [CrossRef]
- Kajimoto, H.; Nakazawa, M.; Murasaki, K.; Hagiwara, N.; Nakanishi, T. Increased P-selectin expression on platelets and decreased plasma thrombomodulin in Fontan patients. Circ. J. 2009, 73, 1705–1710. [Google Scholar] [CrossRef] [Green Version]
- Tomkiewicz-Pajak, L.; Hoffman, P.; Trojnarska, O.; Lipczynska, M.; Podolec, P.; Undas, A. Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J. Thorac. Cardiovasc. Surg. 2014, 147, 1284–1290. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Dhar, A.; Thursz, M.R. The role of hypercoagulability in liver fibrogenesis. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 526–533. [Google Scholar] [CrossRef]
- Ofei, S.; Gariepy, C. When the Cause of Liver Disease Is the Heart. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 3–7. [Google Scholar] [CrossRef]


| All Patients (n = 85) | Thromboembolic Event | p Value | ||
|---|---|---|---|---|
| Yes (n = 16) | No (n = 69) | |||
| Age at most recent evaluation (years) | 27.7 ± 8.2 | 33.8 ± 11.7 | 26.3 ± 6.5 | 0.03 |
| Time since Fontan (years) | 19.3 ± 5.7 | 22.1 ± 5.8 | 18.7 ± 5.6 | 0.08 |
| Gender (female) | 47 (55%) | 9 (56%) | 38 (55%) | 0.93 |
| Most recent BMI (kg/m2) | 24.2 ± 5.1 | 27.2 ± 4.4 | 24.2 ± 5.3 | 0.86 |
| Cardiac diagnosis | 0.73 | |||
| Tricuspid atresia | 26 (31%) | 4 (25%) | 22 (32%) | |
| Double-inlet left ventricle | 11 (13%) | 1 (6%) | 10 (15%) | |
| HLHS | 21 (25%) | 5 (32%) | 16 (23%) | |
| Unbalanced AV canal | 11 (12%) | 1 (6%) | 10 (15%) | |
| Double-outlet right ventricle | 2 (2%) | 1 (6%) | 1 (1%) | |
| Complex two ventricles | 6 (7%) | 2 (13%) | 4 (6%) | |
| Pulmonary atresia/IVS | 3 (4%) | 0 (0%) | 3 (4%) | |
| Mitral atresia | 3 (4%) | 1 (6%) | 2 (3%) | |
| Ebstein anomaly | 2 (2%) | 1 (6%) | 1 (1%) | |
| Type of Fontan circulation | 0.11 | |||
| Atriopulmonary Fontan | 17 (16%) | 6 (38%) | 11 (16%) | |
| Lateral tunnel | 43 (54%) | 9 (56%) | 34 (49%) | |
| Extracardiac conduit | 25 (30%) | 1 (6%) | 24 (35%) | |
| Dominant ventricular morphology | 0.76 | |||
| Left ventricle | 50 (59%) | 9 (56%) | 41 (60%) | |
| Right ventricle | 35(41%) | 7 (44%) | 27 (40%) | |
| History of arrhythmia | 31 (36%) | 11 (69%) | 20 (29%) | 0.004 |
| Protein losing enteropathy | 3 (4%) | 1 (6 %) | 2 (3%) | 0.72 |
| NYHA class | 0.74 | |||
| Class I | 41 (59%) | 9 (56%) | 40 (57%) | |
| Class II | 25 (35%) | 5 (31%) | 25 (36%) | |
| Class III | 4 (6%) | 2 (13%) | 4 (7%) | |
| Anticoagulation at most recent evaluation (warfarin or direct oral anticoagulation) | 30 (35%) | 11 (69%) | 19 (28%) | 0.001 |
| Aspirin at most recent evaluation | 55 (65%) | 5 (31%) | 50 (72%) | 0.001 |
| Number of Patients with Each Test | All Patients (n = 85) | Thromboembolic Events | p Value | ||
|---|---|---|---|---|---|
| Yes (n = 16) | No (n = 69) | ||||
| Ejection fraction (%, CMR) | 50 | 50 ± 8 | 48 ± 5 | 51 ± 9 | 0.14 |
| End diastolic volume (mL/m2, CMR) | 50 | 98 ± 30 | 91 ± 14 | 99 ± 33 | 0.83 |
| End systolic volume (mL/m2, CMR) | 50 | 51 ± 24 | 48 ± 9 | 52 ± 26 | 0.56 |
| At least moderate atrioventricular valve regurgitation (CMR/Echo) | 85 | 11 (13%) | 5 (30%) | 6 (9%) | 0.03 |
| Fontan pressure (mm Hg) | 58 | 13.6 ± 3.9 | 13.9 ± 2.9 | 13.5 ± 4.2 | 0.48 |
| Ventricular end diastolic pressure (mm Hg) | 58 | 10.6 ± 3.8 | 11.1 ± 3.6 | 10.4 ± 2.9 | 0.37 |
| Pulmonary vascular resistance (iWu) | 58 | 1.5 ± 0.9 | 1.5 ± 0.6 | 1.6 ± 0.9 | 0.93 |
| Aortic saturation (%) | 58 | 91 ± 5 | 91 ± 6 | 91 ± 5 | 0.75 |
| Peak VO2 (mL/kg/min) | 73 | 21.2 ± 6.4 | 21.1 ± 8.5 | 22.6 ± 6.0 | 0.57 |
| % predicted VO2 | 73 | 50.1 ± 15.5 | 63.5 ± 12.7 | 59.4 ± 15.9 | 0.36 |
| VE/VCO2 slope | 73 | 37.7 ± 7.5 | 35.9 ± 7.0 | 38.1 ± 7.6 | 0.30 |
| Number of Patients with Results | All Patients (n = 85) | Thromboembolic Events | p Value | ||
|---|---|---|---|---|---|
| Yes (n = 16) | No (n = 69) | ||||
| MRE liver stiffness (kPa) | 70 | 4.4 ± 1.0 | 5.1 ± 1.4 | 4.3 ± 1.2 | 0.04 |
| US liver stiffness (m/s) | 23 | 2.5 ± 0.5 | 2.8 ± 0.4 | 2.4 ± 0.5 | 0.04 |
| History of ascites | 85 | 18 (21%) | 8 (50%) | 10 (15%) | 0.01 |
| Splenomegaly | 85 | 19 (23%) | 4 (27%) | 15 (22%) | 0.68 |
| Portosystemic shunt (varices) | 80 | 13 (16%) | 2 (14%) | 11 (17%) | 0.82 |
| Alanine aminotransferase (unit/L) | 79 | 37 ± 18 | 43 ± 24 | 35 ± 16 | 0.22 |
| Aspartate aminotransferase (unit/L) | 79 | 26 ± 10 | 29 ± 14 | 25 ± 9 | 0.37 |
| Total bilirubin (mg/dL) | 68 | 0.93 ± 0.65 | 0.96 ± 0.81 | 0.93 ± 0.61 | 0.87 |
| Gamma glutamyl transferase (unit/L) | 79 | 100 ± 100 | 142 ± 126 | 84 ± 86 | 0.01 |
| Total protein (gm/dL) | 79 | 7.8 ± 0.8 | 7.7 ± 1.3 | 7.8 ± 0.7 | 0.77 |
| Albumin (gm/dL) | 79 | 4.3 ± 0.5 | 4.2 ± 0.7 | 4.2 ± 0.4 | 0.91 |
| Platelet count(K/mcL) | 80 | 191 ± 71 | 192 ± 73 | 186 ± 63 | 0.85 |
| Predictor | Odds Ratio (95% Confidence Interval) or Parameter Estimate ± SE | p Value |
|---|---|---|
| Univariate analysis | ||
| Age at most recent evaluation (n = 85) | 0.09 ± 0.03 1 | 0.03 |
| Atriopulmonary Fontan (n = 85) | 3.22 (0.95–10.5) 2 | 0.06 |
| History of arrhythmia (n = 85) | 5.39 (1.66–17.51) 2 | 0.004 |
| At least moderate atrioventricular valve regurgitation (n = 85) | 4.83 (1.23–18.89) 2 | 0.03 |
| MRE liver stiffness (n = 70) | 0.77 ± 0.32 1 | 0.02 |
| Liver ultrasound SWE (n = 23) * | 1.50 ± 0.99 1 | 0.10 |
| History of ascites (n = 85) | 5.27 (1.63–17.03) 2 | 0.01 |
| Gamma glutamyl transferase (79) | 0.004 ± 0.002 1 | 0.07 |
| Multivariable analysis (n = 70) | ||
| Age at most recent evaluation | 1.11 (1.02–1.20) 2 | 0.03 |
| MRE liver stiffness | 2.12 (1.08–4.16) 2 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaied, T.; Possner, M.; Lubert, A.M.; Trout, A.T.; Gandhi, J.P.; Garr, B.; Palumbo, J.S.; Palermo, J.J.; Lorts, A.; Veldtman, G.R.; et al. Thromboembolic Events Are Independently Associated with Liver Stiffness in Patients with Fontan Circulation. J. Clin. Med. 2020, 9, 418. https://doi.org/10.3390/jcm9020418
Alsaied T, Possner M, Lubert AM, Trout AT, Gandhi JP, Garr B, Palumbo JS, Palermo JJ, Lorts A, Veldtman GR, et al. Thromboembolic Events Are Independently Associated with Liver Stiffness in Patients with Fontan Circulation. Journal of Clinical Medicine. 2020; 9(2):418. https://doi.org/10.3390/jcm9020418
Chicago/Turabian StyleAlsaied, Tarek, Mathias Possner, Adam M. Lubert, Andrew T. Trout, Janvi P. Gandhi, BreAnn Garr, Joseph S. Palumbo, Joseph J. Palermo, Angela Lorts, Gruschen R. Veldtman, and et al. 2020. "Thromboembolic Events Are Independently Associated with Liver Stiffness in Patients with Fontan Circulation" Journal of Clinical Medicine 9, no. 2: 418. https://doi.org/10.3390/jcm9020418





