The Right Ventricle—You May Forget It, But It Will Not Forget You
Abstract
:1. Introduction
2. Key Pathophysiology of Right Ventricular Failure
2.1. The Right Ventricle is Volume-Tolerant and Pressure-Intolerant
2.2. The Right and Left Ventricles are Inseparably Coupled
2.3. Cardiorespiratory Interactions and the Importance of Spontaneous Respiration in RV Failure
2.4. Right Ventricular Dysfunction—Acute versus Chronic
3. Challenges in the Preoperative Setting
3.1. Pulmonary Hypertension as an Underestimated and Relevant Perioperative Risk Factor
3.2. Diagnostic Strategy for the Workup of Suspected Pulmonary Hypertension
3.3. Risk Stratification—The Balance Between Operative Indication and Perioperative Risk
3.3.1. The Role of Biomarkers
3.3.2. The Role of Echocardiography
3.4. Formulating a Multidisciplinary Perioperative Treatment Strategy
4. Challenges in the Intraoperative and Postoperative Settings
4.1. Prevention of Right Ventricular Failure
4.2. Recognition of Right Ventricular Failure
4.2.1. Choice of Diagnostic Modality
4.2.2. Echocardiography
- -
- Signs of RV dilation: D-shaping, increased RV:LV ratio, tricuspid regurgitation
- -
- Signs of impaired RV systolic function: reduced tricuspid annular plane systolic excursion (TAPSE)
- -
- Signs of elevated RV preload (plethoric inferior vena cava [IVC])
- -
- Vena contracta (VC) width: The width of the regurgitant jet is measured as it leaves the regurgitant orifice. It is best imaged perpendicular to the valve plane, e.g., in the apical 4-chamber view, using CFD with a Nyquist limit of 50–60 cm/s. The VC width corresponds to the width of the narrowest portion of the jet (the “neck), with values > 7 mm suggestive of severe TR.
- -
- Proximal isovelocity surface area (PISA): [46] With significant TR, there is a flow acceleration proximal to the regurgitant valve orifice. This proximal flow convergence occurs along concentric hemispheres and can be visualized using CFD with an adequately set Nyquist limit (15–40 cm/s) to maximize delineation of the flow acceleration. The radius of the PISA correlates with TR severity, with values > 9 mm suggestive of severe TR.
4.2.3. Pulmonary Artery Catheter
- -
- Mean pulmonary artery pressure (mPAP)
- -
- Cardiac output (CO)
- -
- Pulmonary artery wedge pressure (PAWP), also known as pulmonary artery occlusion pressure (PAOP)
- -
- Pre-capillary PH: mPAP > 20 mmHg, PAWP ≤ 15 mmHg, PVR ≥ 3 Woods unitsTypical causes: pulmonary artery hypertension (PAH), lung disease and/or hypoxia, pulmonary artery obstruction
- -
- Isolated post-capillary PH: mPAP > 20 mmHg, PAWP > 15 mmHg, PVR < 3 Woods unitsTypical causes: left heart disease
- -
- Combined pre- and post-capillary PH: mPAP > 20 mmHg, PAWP > 15 mmHg, PVR ≥ 3 Woods unitsTypical causes: left heart disease as well as unclear and/or multifactorial mechanisms
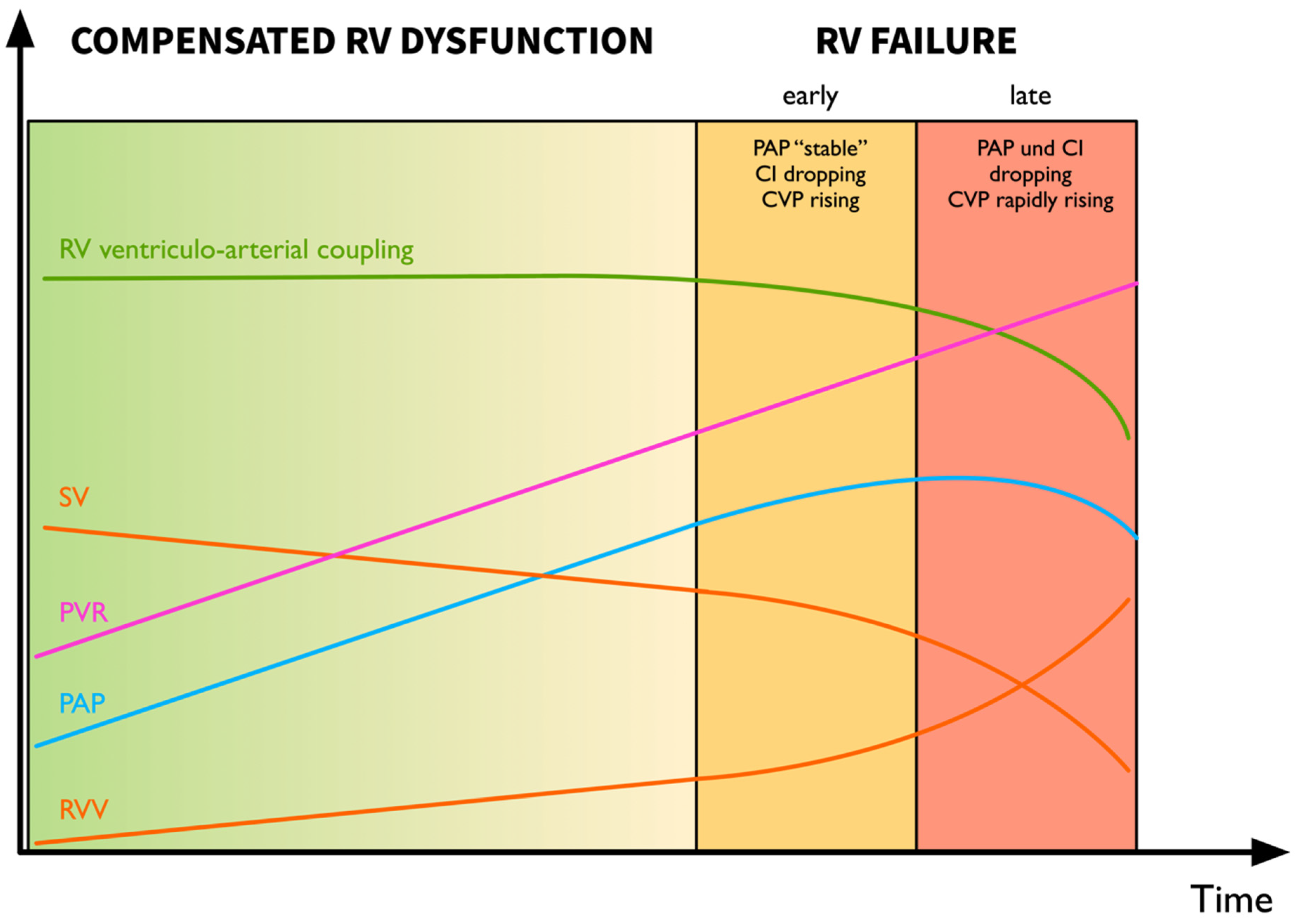
4.2.4. Recognition of the Decompensating Right Ventricle—Pressure Does Not Equal Flow
4.3. Management of RV Failure
4.3.1. Immediate Measures to Break out of the Spiral of RV Failure
- -
- Hypoxia & hypercarbia: Careful titration of PEEP can prove helpful in optimizing gas exchange and can lead to a reduction in PVR. In non-intubated patients, careful application of non-invasive ventilation (NIV) with titration of PEEP to gas exchange and hemodynamics can help delay intubation and enable the treatment of underlying causes. Another potentially promising alternative to intubation is the use of high-flow nasal cannula (HFNC) which enables optimization of oxygenation with often superior patient tolerance and less cardiovascular sequelae compared to NIV [58].
- -
- Increased intrathoracic pressure: in the mechanically ventilated patient, care must be taken to minimize intrathoracic pressures (plateau < 27 mmHg [59]) and tidal volumes.
- -
- Increased sympathetic tone (avoid stress through adequate analgosedation, normothermia)
4.3.2. Treatment of the Underlying Cause
- -
- Progression of pre-existing pulmonary hypertension: removal of exacerbating factors, selective pulmonary vasodilation
- -
- Embolic events: pulmonary embolism (Figure 7), fat embolism, bone cement embolism, amniotic fluid embolism, air/gas embolism, etc: thrombolysis if applicable, supportive measures, selective pulmonary vasodilation
- -
- LV backwards failure due to large myocardial infarction and/or severe mitral regurgitation: recompensation of heart failure, mechanical circulatory support, surgical options
- -
- RV myocardial infarction: coronary revascularization if indicated (ST-elevation myocardial infarction [STEMI] and/or cardiogenic shock), supportive measures, mechanical circulatory support
4.3.3. Management of Volemia in RV failure
4.3.4. Support of Inotropy in RV Failure
4.3.5. Arrhythmias in RV Failure
4.3.6. Anesthetic Induction in the Patient with Right Ventricular Failure
4.3.7. Rescue Strategies for Refractory RV Failure
5. Conclusions
Funding
Conflicts of Interest
References
- Vollema, M.E.; Amanullah, M.R.; Ng, A.; van der Bijl, P.; Prevedello, F.; Sin, Y.; Prihadi, E.A.; Marsan, N.; Ding, Z.; Généreux, P.; et al. Staging Cardiac Damage in Patients With Symptomatic Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2019, 74, 538–549. [Google Scholar] [CrossRef]
- Boissier, F.; Katsahian, S.; Razazi, K.; Thille, A.W.; Roche-Campo, F.; Leon, R.; Vivier, E.; Brochard, L.; Vieillard-Baron, A.; Brun-Buisson, C.; et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- ten Wolde, M.; Söhne, M.; Quak, E.; Gillavry, M.R.; Büller, H.R. Prognostic Value of Echocardiographically Assessed Right Ventricular Dysfunction in Patients With Pulmonary Embolism. Arch. Intern. Med. 2004, 164, 1685–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishna, G.; Sprung, J.; Ravi, B.S.; Chandrasekaran, K.; McGoon, M.D. Impact of Pulmonary Hypertension on the Outcomes of Noncardiac Surgery Predictors of Perioperative Morbidity and Mortality. J. Am. Coll. Cardiol. 2005, 45, 1691–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaw, R.; Pasupuleti, V.; Deshpande, A.; Hamieh, T.; Walker, E.; Minai, O.A. Pulmonary hypertension: An important predictor of outcomes in patients undergoing non-cardiac surgery. Resp. Med. 2011, 105, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Markin, N.W.; Gmelch, B.S.; Griffee, M.J.; Holmberg, T.J.; Morgan, D.E.; Zimmerman, J.M. A Review of 364 Perioperative Rescue Echocardiograms: Findings of an Anesthesiologist-Staffed Perioperative Echocardiography Service. J. Cardiothor. Vasc. Anesth. 2015, 29, 82–88. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right Ventricular Function in Cardiovascular Disease, Part I. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right Ventricular Function in Cardiovascular Disease, Part II. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef]
- Damiano, R.; Follette, L.P.; Cox, J.; Lowe, J.; Santamore, W. Significant left ventricular contribution to right ventricular systolic function. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H1514–H1524. [Google Scholar] [CrossRef]
- Santamore, W.P.; Gray, L. Significant Left Ventricular Contributions to Right Ventricular Systolic Function Mechanism and Clinical Implications. Chest 1995, 107, 1134–1145. [Google Scholar] [CrossRef]
- Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wanner, P.M.; Filipovic, M. Der rechte Ventrikel—Das Wichtigste für den Intensivmediziner. In Intensivmedizin - Kompendium und Repetitorium zur interdisziplinären Weiter- und Fortbildung; Eckart, J., Forst, H., Briegel, J., Eds.; Ecomed Verlagsgesellschaft AG & Co: Landsberg, Germany, 2018. [Google Scholar]
- Hoeper, M.M.; Galiè, N.; Murali, S.; Olschewski, H.; Rubenfire, M.; Robbins, I.M.; Farber, H.W.; McLaughlin, V.; Shapiro, S.; Pepke-Zaba, J.; et al. Outcome after Cardiopulmonary Resuscitation in Patients with Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2002, 165, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.; Whittingham, H.; Tomlinson, G.; Granton, J. Utility of the Physical Examination in Detecting Pulmonary Hypertension. A Mixed Methods Study. PLoS ONE 2014, 9, e108499. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertensionThe Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Hur, D.J.; Sugeng, L. Non-invasive Multimodality Cardiovascular Imaging of the Right Heart and Pulmonary Circulation in Pulmonary Hypertension. Front. Cardiovasc. Med. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauger, C.; Gilbert, K.; Lee, A.M.; Sanghvi, M.M.; Aung, N.; Fung, K.; Carapella, V.; Piechnik, S.K.; Neubauer, S.; Petersen, S.E.; et al. Right ventricular shape and function: Cardiovascular magnetic resonance reference morphology and biventricular risk factor morphometrics in UK Biobank. J. Cardiov. Magn. Reson. 2019, 21, 41. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; Hert, S.D.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar]
- Duceppe, E.; Parlow, J.; MacDonald, P.; Lyons, K.; McMullen, M.; nathan, S.; Graham, M.; Tandon, V.; Styles, K.; Bessissow, A.; et al. Canadian Cardiovascular Society Guidelines on Perioperative Cardiac Risk Assessment and Management for Patients Who Undergo Noncardiac Surgery. Can. J. Cardiol. 2017, 33, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Rodseth, R.N.; Biccard, B.M.; Manach, Y.; Sessler, D.I.; Buse, G.A.; Thabane, L.; Schutt, R.C.; Bolliger, D.; Cagini, L.; Cardinale, D.; et al. The Prognostic Value of Pre-Operative and Post-Operative B-Type Natriuretic Peptides in Patients Undergoing Noncardiac Surgery B-Type Natriuretic Peptide and N-Terminal Fragment of Pro-B-Type Natriuretic Peptide: A Systematic Review and Individual Patient Data Meta-Analysis. J. Am. Coll. Cardiol. 2014, 63, 170–180. [Google Scholar]
- Andreassen, A.K.; Wergeland, R.; Simonsen, S.; Geiran, O.; Guevara, C.; Ueland, T. N-Terminal Pro-B-Type Natriuretic Peptide as an Indicator of Disease Severity in a Heterogeneous Group of Patients With Chronic Precapillary Pulmonary Hypertension. Am. J. Cardiol. 2006, 98, 525–529. [Google Scholar] [CrossRef]
- Nagaya, N.; Nishikimi, T.; Uematsu, M.; Satoh, T.; Kyotani, S.; Sakamaki, F.; Kakishita, M.; Fukushima, K.; Okano, Y.; Nakanishi, N.; et al. Plasma Brain Natriuretic Peptide as a Prognostic Indicator in Patients With Primary Pulmonary Hypertension. Circulation 2000, 102, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijalkowska, A.; Kurzyna, M.; Torbicki, A.; Szewczyk, G.; Florczyk, M.; Pruszczyk, P.; Szturmowicz, M. Serum N-Terminal Brain Natriuretic Peptide as a Prognostic Parameter in Patients With Pulmonary Hypertension. Chest 2006, 129, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztrymf, B.; Souza, R.; Bertoletti, L.; Jais, X.; Sitbon, O.; Price, L.; Simonneau, G.; Humbert, M. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur. Respir. J. 2009, 35, 1286–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klok, F.A.; Mos, I.C.; Huisman, M.V. Brain-Type Natriuretic Peptide Levels in the Prediction of Adverse Outcome in Patients with Pulmonary Embolism. Am. J. Resp. Crit. Care Med. 2008, 178, 425–430. [Google Scholar] [CrossRef]
- Coutance, G.; Cauderlier, E.; Ehtisham, J.; Hamon, M.; Hamon, M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: A meta-analysis. Crit. Care 2011, 15, R103. [Google Scholar] [CrossRef] [Green Version]
- Buse, G.A.; Koller, M.T.; Burkhart, C.; Seeberger, M.D.; Filipovic, M. The Predictive Value of Preoperative Natriuretic Peptide Concentrations in Adults Undergoing Surgery. Anesth. Analg. 2011, 112, 1019–1033. [Google Scholar] [CrossRef]
- Rohde, L.E.; Polanczyk, C.A.; Goldman, L.; Cook, E.F.; Lee, R.T.; Lee, T.H. Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgery. Am. J. Cardiol. 2001, 87, 505–509. [Google Scholar] [CrossRef]
- Park, S.-J.; Choi, J.-H.; Cho, S.-J.; Chang, S.-A.; Choi, J.-O.; Lee, S.-C.; Park, S.; Oh, J.K.; Kim, D.-K.; Jeon, E.-S. Comparison of Transthoracic Echocardiography With N-Terminal Pro-Brain Natriuretic Peptide as a Tool for Risk Stratification of Patients Undergoing Major Noncardiac Surgery. Korean Circ. J. 2011, 41, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Wijeysundera, D.N.; Beattie, S.W.; Karkouti, K.; Neuman, M.D.; Austin, P.C.; Laupacis, A. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: Population based cohort study. BMJ 2011, 342, d3695. [Google Scholar] [CrossRef] [Green Version]
- Bolat, İ. Preoperative Right Ventricular Echocardiographic Parameters Predict Perioperative Cardiovascular Complications in Patients Undergoing Non-Cardiac Surgery. Heart Lung Circ. 2019. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Choudhary, G.; Lakshmanadoss, U.; Prasad, H.; Shah, A.; Babayan, Z.; Stapleton, D. Poor Right Ventricular Systolic Function (Lower TAPSE) and Higher Pulmonary Artery Systolic Pressure (PASP) Predicts Early Readmissions and All Cause Mortality in Elderly Patients with Heart Failure. J. Card. Fail. 2014, 7 (Suppl. S1), A307. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R.; Arena, R.; Corrà, U.; Ghio, S.; Forfia, P.; Rossi, A.; Cahalin, L.P.; Bandera, F.; Temporelli, P. Echocardiography of Right Ventriculoarterial Coupling Combined With Cardiopulmonary Exercise Testing to Predict Outcome in Heart Failure. Chest 2015, 148, 226–234. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Atkinson, T.M.; Giraud, G.D.; Togioka, B.M.; Jones, D.B.; Cigarroa, J.E. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017, 135, 700–710. [Google Scholar] [CrossRef]
- Ghai, B.; Mohan, V.; Khetarpal, M.; Malhotra, N. Epidural anesthesia for cesarean section in a patient with Eisenmenger’s syndrome. Int. J. Obstet. Anesth. 2002, 11, 44–47. [Google Scholar] [CrossRef]
- Chen, L.-K.; Chen, S.-Y. Ultra-low dose combined spinal-epidural anaesthesia for Caesarean section in severe pulmonary hypertension induced by Ritodrine. BJA Br. J. Anaesth. 2010, 105. [Google Scholar] [CrossRef]
- Mafra, A.; Conde, R.; Castro, A.; Pereira, M.; Pinheiro, C.; Rodrigues, M. Continuous spinal anesthesia in a patient with severe pulmonary hypertension—A clinical case. Eur. J. Anaesth. 2013, 30, 132. [Google Scholar] [CrossRef]
- Seif, D.; Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. Bedside Ultrasound in Resuscitation and the Rapid Ultrasound in Shock Protocol. Crit. Care Res. Pract. 2012, 2012, 503254. [Google Scholar] [CrossRef] [Green Version]
- Noordegraaf, A.; Chin, K.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.; Kawut, S.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiog. 2010, 23, 685–713. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Hear J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.; Badano, L.; Zamorano, J.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.A. Proximal Isovelocity Surface Area Should Be Routinely Measured in Evaluating Mitral Regurgitation: A Core Review. Anesth. Analg. 2007, 105, 940–943. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiog. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- McClanahan, A.; Guglin, M. Right Ventricular Dysfunction Compromises Accuracy of Echocardiographic Diagnosis of Pulmonary Hypertension in Heart Failure. J. Card. Fail. 2011, 17, 1023–1027. [Google Scholar] [CrossRef]
- Sato, T.; Tsujino, I.; Ohira, H.; Oyama-Manabe, N.; Yamada, A.; Ito, Y.M.; Goto, C.; Watanabe, T.; Sakaue, S.; Nishimura, M. Validation Study on the Accuracy of Echocardiographic Measurements of Right Ventricular Systolic Function in Pulmonary Hypertension. J. Am. Soc. Echocardiog. 2012, 25, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Magunia, H.; Schmid, E.; Hilberath, J.N.; Häberle, L.; Grasshoff, C.; Schlensak, C.; Rosenberger, P.; Nowak-Machen, M. 2D Echocardiographic Evaluation of Right Ventricular Function Correlates With 3D Volumetric Models in Cardiac Surgery Patients. J. Cardiothor. Vasc. Anesth. 2017, 31, 595–601. [Google Scholar] [CrossRef]
- Schneider, M.; Ran, H.; Aschauer, S.; Binder, C.; Mascherbauer, J.; Lang, I.; Hengstenberg, C.; Goliasch, G.; Binder, T. Visual assessment of right ventricular function by echocardiography: How good are we? Int. J. Cardiovasc. Imaging 2019, 35, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Obuchowski, N.A.; Rodriguez, L.; Popovic, Z.; Kwon, D.; Marwick, T.H. Accuracy and Interobserver Concordance of Echocardiographic Assessment of Right Ventricular Size and Systolic Function: A Quality Control Exercise. J. Am. Soc. Echocardiog. 2012, 25, 709–713. [Google Scholar] [CrossRef]
- Rhodes, A.; Cusack, R.J.; Newman, P.J.; Grounds, M.R.; Bennett, D.E. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med. 2002, 28, 256–264. [Google Scholar] [CrossRef]
- Richard, C.; Warszawski, J.; Anguel, N.; Deye, N.; Combes, A.; Barnoud, D.; Boulain, T.; Lefort, Y.; Fartoukh, M.; Baud, F.; et al. Early Use of the Pulmonary Artery Catheter and Outcomes in Patients With Shock and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 2003, 290, 2713–2720. [Google Scholar] [CrossRef]
- Harvey, S.; Harrison, D.A.; Singer, M.; Ashcroft, J.; Jones, C.M.; Elbourne, D.; Brampton, W.; Williams, D.; Young, D.; Rowan, K.; et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet 2005, 366, 472–477. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Preston, I.R. Right heart catheterisation: Best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Gupta, B.; Kerai, S.; Kakkar, K.; Gupta, L. Role of High-flow Nasal Oxygen Therapy in Cases with Pulmonary Hypertension in an Intensive Care Unit Setting. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2019, 23, 458–461. [Google Scholar] [CrossRef]
- Jardin, F.; Vieillard-Baron, A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intens. Care Med. 2007, 33, 444–447. [Google Scholar] [CrossRef]
- Winterhalter, M.; Simon, A.; Fischer, S.; Rahe-Meyer, N.; Chamtzidou, N.; Hecker, H.; Zuk, J.; Piepenbrock, S.; Strüber, M. Comparison of Inhaled Iloprost and Nitric Oxide in Patients With Pulmonary Hypertension During Weaning From Cardiopulmonary Bypass in Cardiac Surgery: A Prospective Randomized Trial. J. Cardiothor. Vasc. Anesth. 2008, 22, 406–413. [Google Scholar] [CrossRef]
- Krug, S. Inhaled iloprost for the control of pulmonary hypertension. Vasc. Heal. Risk Manag. 2009, 5, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Denault, A.Y.; Bussières, J.S.; Arellano, R.; Finegan, B.; Gavra, P.; Haddad, F.; Nguyen, A.Q.; Varin, F.; Fortier, A.; Levesque, S.; et al. A multicentre randomized-controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Can. J. Anesth. J. Can. D’anesthésie 2016, 63, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Wort, S.J.; Finney, S.J.; Marino, P.S.; Brett, S.J. Pulmonary vascular and right ventricular dysfunction in adult critical care: Current and emerging options for management: A systematic literature review. Crit. Care 2010, 14, R169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michard, F.; Richards, G.; Biais, M.; Lopes, M.; Auler, J. Using pulse pressure variation or stroke volume variation to diagnose right ventricular failure? Crit. Care Lond. Engl. 2010, 14, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P. Fluid therapy in 2015 and beyond: The mini-fluid challenge and mini-fluid bolus approach. BJA Br. J. Anaesth. 2015, 115, 347–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, L.J.; Rooney, M.W.; Wat, S.S.; Kleinmann, B.; Mathru, M. Norepinephrine and Phenylephrine Effects on Right Ventricular Function in Experimental Canine Pulmonary Embolism. Chest 1991, 100, 796–801. [Google Scholar] [CrossRef] [Green Version]
- Kwak, Y.; Lee, C.; Park, Y.; Hong, Y. The effect of phenylephrine and norepinephrine in patients with chronic pulmonary hypertension*. Anaesthesia 2002, 57, 9–14. [Google Scholar] [CrossRef]
- Eichhorn, E.J.; Konstam, M.A.; Weiland, D.S.; Roberts, D.J.; Martin, T.T.; Stransky, N.B.; Salem, D.N. Differential effects of milrinone and dobutamine on right ventricular preload, afterload and systolic performance in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1987, 60, 1329–1333. [Google Scholar] [CrossRef]
- Hansen, M.; Andersen, A.; Nielsen-Kudsk, J. Levosimendan in pulmonary hypertension and right heart failure. Pulm. Circ. 2018, 8, 2045894018790905. [Google Scholar] [CrossRef] [Green Version]
- Léopold, V.; Gayat, E.; Pirracchio, R.; Spinar, J.; Parenica, J.; Tarvasmäki, T.; Lassus, J.; Harjola, V.-P.; Champion, S.; Zannad, F.; et al. Epinephrine and short-term survival in cardiogenic shock: An individual data meta-analysis of 2583 patients. Intensive Care Med. 2018, 44, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Jia, L.; Hao, Y.; Huang, S.; Ma, Y.; Li, X.; Wang, M.; Mao, Y. Efficacy and safety of levosimendan in patients with acute right heart failure: A meta-analysis. Life Sci. 2017, 184, 30–36. [Google Scholar] [CrossRef]
- Miller, M.; Kruit, N.; Heldreich, C.; Ware, S.; Habig, K.; Reid, C.; Burns, B. Hemodynamic Response After Rapid Sequence Induction With Ketamine in Out-of-Hospital Patients at Risk of Shock as Defined by the Shock Index. Ann. Emerg. Med. 2016, 68, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, E.; Frazier, J.W.; Leder, M.; Fraser, D.D.; Tobias, J.D. Cardiac Arrest Following Ketamine Administration for Rapid Sequence Intubation. J. Intensive Care Med. 2012, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.; Burkhoff, D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, N.; Dickert, N.W.; Samady, H.; Babaliaros, V. The use of hemodynamic support in massive pulmonary embolism. Catheter. Cardio. Interv. 2017, 90, 516–520. [Google Scholar] [CrossRef]
- Shokr, M.; Rashed, A.; Mostafa, A.; Mohamad, T.; Schreiber, T.; Elder, M.; Kaki, A. Impella RP Support and Catheter-Directed Thrombolysis to Treat Right Ventricular Failure Caused by Pulmonary Embolism in 2 Patients. Tex. Heart Inst. J. 2018, 45, 182–185. [Google Scholar] [CrossRef] [Green Version]
- Members, A.; Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef] [Green Version]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, O.J.; Ho, J.K.; Yezbick, A.B.; Sanchez, E.; Singh, V.; Mahajan, A. Right Ventricular Failure Resulting from Pressure Overload: Role of Intra-Aortic Balloon Counterpulsation and Vasopressor Therapy. J. Surg. Res. 2010, 164, 58–66. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; DeVore, A.D.; Sun, J.-L.; Barnett, A.S.; Samsky, M.D.; Shaw, L.K.; Chiswell, K.; Patel, C.B.; Patel, M.R. The impact of a failing right heart in patients supported by intra-aortic balloon counterpulsation. Eur. Heart J. Acute Cardiovasc. Care 2016, 6, 709–718. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanner, P.M.; Filipovic, M. The Right Ventricle—You May Forget It, But It Will Not Forget You. J. Clin. Med. 2020, 9, 432. https://doi.org/10.3390/jcm9020432
Wanner PM, Filipovic M. The Right Ventricle—You May Forget It, But It Will Not Forget You. Journal of Clinical Medicine. 2020; 9(2):432. https://doi.org/10.3390/jcm9020432
Chicago/Turabian StyleWanner, Patrick M., and Miodrag Filipovic. 2020. "The Right Ventricle—You May Forget It, But It Will Not Forget You" Journal of Clinical Medicine 9, no. 2: 432. https://doi.org/10.3390/jcm9020432



