Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation
Abstract
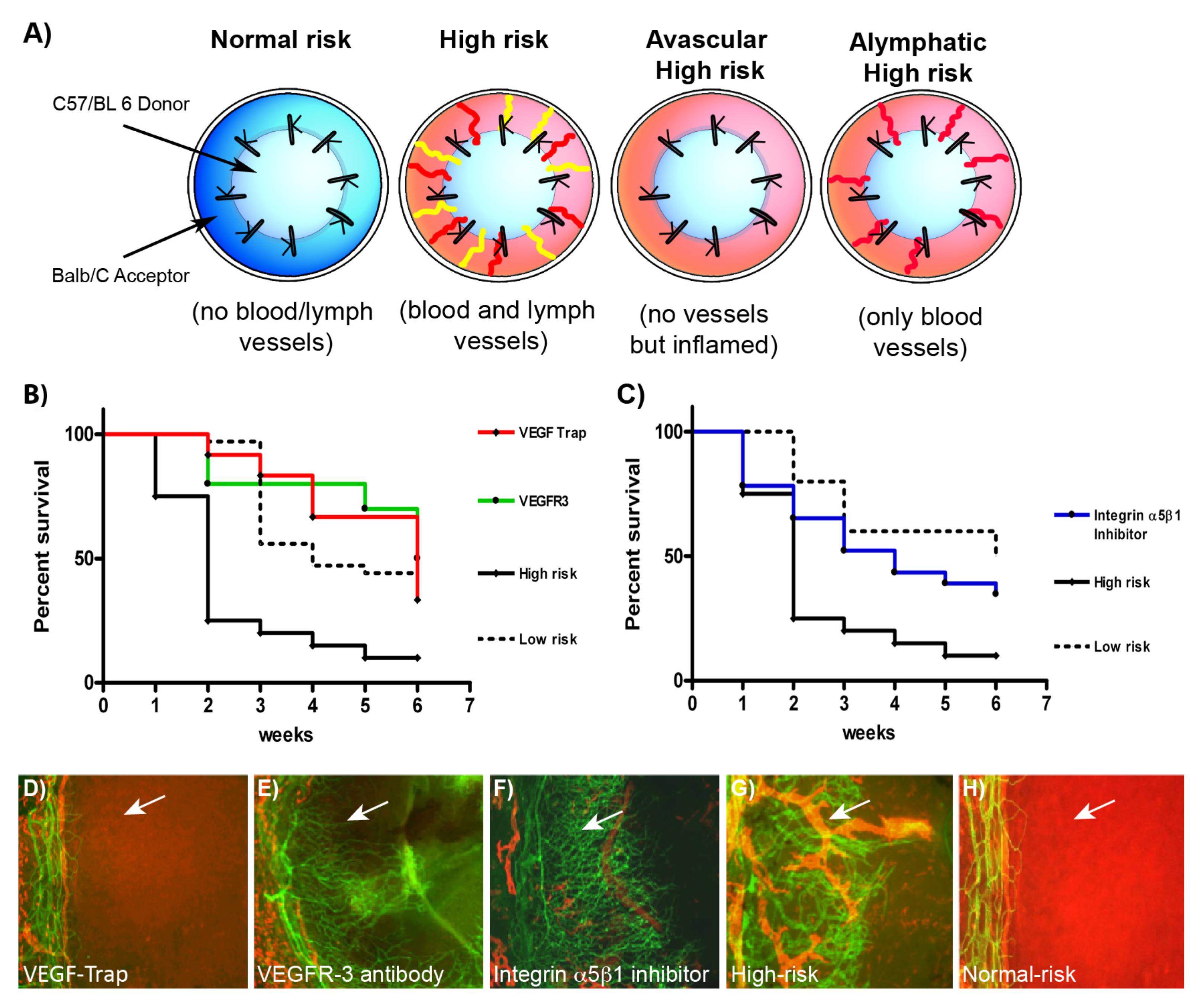
:1. Introduction
2. Endogenous Regulators of (lymph)angiogenesis
3. Endogenous Regulators of Lymphangiogenesis in Corneal Transplantation
4. Identification of Novel Endogenous Regulators of Lymphangiogenesis
4.1. Proteins and Peptides in Lymphangiogenesis
4.2. Non-Coding RNAs in Lymphangiogenesis
5. Murine Cornea is a Suitable Model for Identification of Novel Endogenous Modulators of Lymphangiogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cursiefen, C.; Schlotzer-Schrehardt, U.; Kuchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Invest. Ophthalmol Vis. Sci 2002, 43, 2127–2135. [Google Scholar] [PubMed]
- Chen, L. Ocular lymphatics: State-of-the-art review. Lymphology 2009, 42, 66–76. [Google Scholar] [PubMed]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol 2010, 184, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Oh, S.J.; Jeltsch, M.M.; Birkenhager, R.; McCarthy, J.E.; Weich, H.A.; Christ, B.; Alitalo, K.; Wilting, J. VEGF and VEGF-C: Specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol 1997, 188, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Otrock, Z.K.; Makarem, J.A.; Shamseddine, A.I. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol. Dis 2007, 38, 258–268. [Google Scholar] [CrossRef]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Sheppard, D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J. Biol Chem 2005, 280, 4544–4552. [Google Scholar] [CrossRef] [Green Version]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Bussolino, F.; Albini, A.; Camussi, G.; Presta, M.; Viglietto, G.; Ziche, M.; Persico, G. Role of soluble mediators in angiogenesis. Eur J. Cancer 1996, 32A, 2401–2412. [Google Scholar] [CrossRef]
- Berdugo, M.; Andrieu-Soler, C.; Doat, M.; Courtois, Y.; BenEzra, D.; Behar-Cohen, F. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest. Ophthalmol Vis. Sci 2005, 46, 4072–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2007, 48, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, K.; Li, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Wasi, S.; Ferrara, N.; Orci, L.; Montesano, R. In vitro angiogenic and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res 1994, 210, 298–305. [Google Scholar] [CrossRef]
- Jitariu, A.A.; Cimpean, A.M.; Kundnani, N.R.; Raica, M. Platelet-derived growth factors induced lymphangiogenesis: Evidence, unanswered questions and upcoming challenges. Arch. Med. Sci 2015, 11, 57–66. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Lohela, M.; Morisada, T.; Tornberg, J.; Norrmen, C.; Oike, Y.; Pajusola, K.; Thurston, G.; Suda, T.; et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005, 105, 4642–4648. [Google Scholar] [CrossRef] [Green Version]
- Yuen, D.; Grimaldo, S.; Sessa, R.; Ecoiffier, T.; Truong, T.; Huang, E.; Bernas, M.; Daley, S.; Witte, M.; Chen, L. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest. Ophthalmol Vis. Sci. 2014, 55, 3320–3327. [Google Scholar] [CrossRef] [Green Version]
- Kajiya, K.; Hirakawa, S.; Ma, B.; Drinnenberg, I.; Detmar, M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005, 24, 2885–2895. [Google Scholar] [CrossRef] [Green Version]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu Rev. Physiol 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol 2014, 5, 508. [Google Scholar] [CrossRef] [Green Version]
- Peppicelli, S.; Bianchini, F.; Calorini, L. Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett 2014, 8, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Garcia Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Shin, K.; Kataru, R.P.; Park, H.J.; Kwon, B.I.; Kim, T.W.; Hong, Y.K.; Lee, S.H. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun 2015, 6, 6196. [Google Scholar] [CrossRef] [Green Version]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol Soc. 2006, 104, 264–302. [Google Scholar]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy 2007, 92, 50–57. [Google Scholar] [CrossRef]
- Lawler, J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 2000, 12, 634–640. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Mienaltowski, M.J.; Birk, D.E. Regulation of corneal stroma extracellular matrix assembly. Exp. Eye Res. 2015, 133, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Ishizaki, M.; Shimoda, M.; Wakamatsu, K.; Ogro, T.; Yamanaka, N.; Kao, C.W.; Kao, W.W. Stromal fibroblasts are associated with collagen IV in scar tissues of alkali-burned and lacerated corneas. Curr Eye Res. 1997, 16, 339–348. [Google Scholar] [CrossRef]
- Frikeche, J.; Maiti, G.; Chakravarti, S. Small leucine-rich repeat proteoglycans in corneal inflammation and wound healing. Exp. Eye Res. 2016, 151, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, S.; Mai, K.; McCluskey, P.; Wakefield, D. The role of lumican in ocular disease. ISRN Ophthalmol 2013, 2013, 632302. [Google Scholar] [CrossRef] [Green Version]
- Faye, C.; Moreau, C.; Chautard, E.; Jetne, R.; Fukai, N.; Ruggiero, F.; Humphries, M.J.; Olsen, B.R.; Ricard-Blum, S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol Chem 2009, 284, 22029–22040. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Stenbjorn, R.S.; Gorse, K.; Su, K.; Hauser, K.F.; Ricard-Blum, S.; Pihlajaniemi, T.; Fox, M.A. Target-derived matricryptins organize cerebellar synapse formation through alpha3beta1 integrins. Cell Rep. 2012, 2, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef] [Green Version]
- Bix, G.; Iozzo, R.V. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol 2005, 15, 52–60. [Google Scholar] [CrossRef]
- Mundel, T.M.; Kalluri, R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007, 74, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarui, T.; Miles, L.A.; Takada, Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J. Biol Chem 2001, 276, 39562–39568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troyanovsky, B.; Levchenko, T.; Mansson, G.; Matvijenko, O.; Holmgren, L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol 2001, 152, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest. Ophthalmol Vis. Sci 2004, 45, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef] [Green Version]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef] [Green Version]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol 2014, 252, 1755–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol Vis. Sci 2004, 45, 2666–2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2011, 52, 5778–5785. [Google Scholar] [CrossRef] [Green Version]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.M.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.H.; Saban, D.R.; Okanobo, A.; Nallasamy, N.; Sadrai, Z.; Chauhan, S.K.; Hajrasouliha, A.R.; Dana, R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest. Ophthalmol Vis. Sci 2010, 51, 2411–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.A.; Mammo, D.A.; Page, M.A. Intrastromal bevacizumab in the management of corneal neovascularization: A retrospective review. Graefes Arch. Clin. Exp. Ophthalmol 2019. [Google Scholar] [CrossRef]
- Sarah, B.; Ibtissam, H.; Mohammed, B.; Hasna, S.; Abdeljalil, M. Intrastromal Injection of Bevacizumab in the Management of Corneal Neovascularization: About 25 Eyes. J. Ophthalmol 2016, 2016, 6084270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, S.N.; Lichtinger, A.; Kim, P.; Amiran, M.D.; Slomovic, A.R. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea 2011, 30, 1110–1114. [Google Scholar] [CrossRef]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of topical bevacizumab in high-risk corneal transplant survival. Pak. J. Med. Sci 2013, 29, 519–522. [Google Scholar] [CrossRef]
- Dekaris, I.; Gabric, N.; Draca, N.; Pauk-Gulic, M.; Milicic, N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefes Arch. Clin. Exp. Ophthalmol 2015, 253, 287–294. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol 2009, 247, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of sub-conjunctival and topical bevacizumab in high-risk corneal transplant survival. J. Pak. Med. Assoc. 2013, 63, 1256–1259. [Google Scholar] [PubMed]
- Hos, D.; Le, V.N.H.; Hellmich, M.; Siebelmann, S.; Roters, S.; Bachmann, B.O.; Cursiefen, C. Risk of Corneal Graft Rejection After High-risk Keratoplasty Following Fine-needle Vessel Coagulation of Corneal Neovascularization Combined With Bevacizumab: A Pilot Study. Transplant. Direct 2019, 5, e452. [Google Scholar] [CrossRef] [PubMed]
- White, M.F. The IRS-signaling system: A network of docking proteins that mediate insulin and cytokine action. Recent Prog Horm Res. 1998, 53, 119–138. [Google Scholar]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecova, P.; Levy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The I-CAN study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef] [Green Version]
- Niederkorn, J.Y. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007, 32, 1005–1016. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2012, 53, 1244–1250. [Google Scholar] [CrossRef] [Green Version]
- Acton, S.E.; Astarita, J.L.; Malhotra, D.; Lukacs-Kornek, V.; Franz, B.; Hess, P.R.; Jakus, Z.; Kuligowski, M.; Fletcher, A.L.; Elpek, K.G.; et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 2012, 37, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Colonna, M.; Samaridis, J.; Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J. Immunol 2000, 30, 697–704. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol 2004, 15, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Turley, S.J.; Fletcher, A.L.; Elpek, K.G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol 2010, 10, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Maruyama, K.; Kato, Y.; Kajiya, K.; Moritoh, S.; Yamamoto, K.; Matsumoto, Y.; Sawane, M.; Kerjaschki, D.; Nakazawa, T.; et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest. Ophthalmol Vis. Sci 2014, 55, 4813–4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmy-Susini, B.; Varner, J.A. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res. Biol 2008, 6, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, K.; Watabe, T.; Saito, A.; Yoshimatsu, Y.; Imaizumi, N.; Masui, S.; Hirashima, M.; Morisada, T.; Oike, Y.; Araie, M.; et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol. Biol Cell 2007, 18, 1421–1429. [Google Scholar] [CrossRef]
- Kang, G.J.; Truong, T.; Huang, E.; Su, V.; Ge, S.; Chen, L. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Invest. Ophthalmol Vis. Sci 2016, 57, 5935–5939. [Google Scholar] [CrossRef] [Green Version]
- Garmy-Susini, B.; Makale, M.; Fuster, M.; Varner, J.A. Methods to study lymphatic vessel integrins. Methods Enzymol 2007, 426, 415–438. [Google Scholar] [CrossRef]
- Nasarre, P.; Gemmill, R.M.; Drabkin, H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther 2014, 7, 1663–1687. [Google Scholar] [CrossRef] [Green Version]
- Suto, F.; Ito, K.; Uemura, M.; Shimizu, M.; Shinkawa, Y.; Sanbo, M.; Shinoda, T.; Tsuboi, M.; Takashima, S.; Yagi, T.; et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci 2005, 25, 3628–3637. [Google Scholar] [CrossRef]
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999, 99, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Buehler, A.; Sitaras, N.; Favret, S.; Bucher, F.; Berger, S.; Pielen, A.; Joyal, J.S.; Juan, A.M.; Martin, G.; Schlunck, G.; et al. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett 2013, 587, 1650–1655. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liegl, R.; Gong, Y.; Buhler, A.; Cakir, B.; Meng, S.S.; Burnim, S.B.; Liu, C.H.; Reuer, T.; Zhang, P.; et al. Sema3f Protects Against Subretinal Neovascularization In Vivo. EBioMedicine 2017, 18, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuer, T.; Schneider, A.C.; Cakir, B.; Buhler, A.D.; Walz, J.M.; Lapp, T.; Lange, C.; Agostini, H.; Schlunck, G.; Cursiefen, C.; et al. Semaphorin 3F Modulates Corneal Lymphangiogenesis and Promotes Corneal Graft Survival. Invest. Ophthalmol Vis. Sci 2018, 59, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Heishi, T.; Hosaka, T.; Suzuki, Y.; Miyashita, H.; Oike, Y.; Takahashi, T.; Nakamura, T.; Arioka, S.; Mitsuda, Y.; Takakura, T.; et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol 2010, 176, 1950–1958. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Mak, J.; Pardanaud, L.; Caunt, M.; Kasman, I.; Larrivee, B.; Del Toro, R.; Suchting, S.; Medvinsky, A.; et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol 2010, 188, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Hasegawa, Y.; Yamashita, H.; Shimizu, K.; Ding, Y.; Abe, M.; Ohta, H.; Imagawa, K.; Hojo, K.; Maki, H.; et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J. Clin. Invest. 2004, 114, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.L.; Sun, J.F.; Wang, X.Y.; Du, L.L.; Liu, P. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol. Vis. 2010, 16, 2354–2361. [Google Scholar]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef]
- Barbolina, M.V.; Stack, M.S. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin Cell Dev. Biol 2008, 19, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Han, K.Y.; Dugas-Ford, J.; Lee, H.; Chang, J.H.; Azar, D.T. MMP14 Cleavage of VEGFR1 in the Cornea Leads to a VEGF-Trap Antiangiogenic Effect. Invest. Ophthalmol Vis. Sci 2015, 56, 5450–5456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.L.; Cao, R.; Jin, G.; Chan, K.M.; Cao, Y.; Zhou, Z. When MT1-MMP meets ADAMs. Cell Cycle 2012, 11, 2793–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.L.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun 2016, 7, 10824. [Google Scholar] [CrossRef] [Green Version]
- Du, H.T.; Du, L.L.; Tang, X.L.; Ge, H.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefes Arch. Clin. Exp. Ophthalmol 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Invest. Ophthalmol Vis. Sci 2016, 57, 6554–6560. [Google Scholar] [CrossRef] [Green Version]
- Nacher, J.C. Community structure of non-coding RNA interaction network. J. Integr Bioinform 2013, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Khan, A.; Nasr, P.; El-Charabaty, E.; El-Sayegh, S. An Insight Into the Immunologic Events and Risk Assessment in Renal Transplantation. J. Clin. Med. Res. 2016, 8, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Oghumu, S.; Bracewell, A.; Nori, U.; Maclean, K.H.; Balada-Lasat, J.M.; Brodsky, S.; Pelletier, R.; Henry, M.; Satoskar, A.R.; Nadasdy, T.; et al. Acute pyelonephritis in renal allografts: A new role for microRNAs? Transplantation 2014, 97, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Wu, J.; Ma, M.; Wu, X.; Wen, J.; Yu, J. An integrated deep sequencing analysis of microRNAs in transplanted corneas. Mol. Immunol 2018, 101, 429–439. [Google Scholar] [CrossRef]
- Wang, T.; Li, F.; Geng, W.; Ruan, Q.; Shi, W. MicroRNA-122 ameliorates corneal allograft rejection through the downregulation of its target CPEB1. Cell Death Discov 2017, 3, 17021. [Google Scholar] [CrossRef] [PubMed]
- Grimaldo, S.; Yuen, D.; Theis, J.; Ng, M.; Ecoiffier, T.; Chen, L. MicroRNA-184 Regulates Corneal Lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2015, 56, 7209–7213. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.K.; Detmar, M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003, 314, 85–92. [Google Scholar] [CrossRef]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn 2002, 225, 351–357. [Google Scholar] [CrossRef]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Michael, M.Z.; Harvey, N.L. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 2010, 116, 2395–2401. [Google Scholar] [CrossRef] [Green Version]
- Pedrioli, D.M.; Karpanen, T.; Dabouras, V.; Jurisic, G.; van de Hoek, G.; Shin, J.W.; Marino, D.; Kalin, R.E.; Leidel, S.; Cinelli, P.; et al. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol. Cell Biol 2010, 30, 3620–3634. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Choi, J.S.; Rho, C.R.; Joo, C.K.; Lee, S.K. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J. Biomed. Sci 2015, 22, 3. [Google Scholar] [CrossRef] [Green Version]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flister, M.J.; Wilber, A.; Hall, K.L.; Iwata, C.; Miyazono, K.; Nisato, R.E.; Pepper, M.S.; Zawieja, D.C.; Ran, S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood 2010, 115, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Li, Y.; He, Y.; Xu, Z.; Chen, H.; Min, W. Mirtron microRNA-1236 inhibits VEGFR-3 signaling during inflammatory lymphangiogenesis. Arterioscler Thromb Vasc Biol 2012, 32, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Zawieja, D.C.; Davis, M.J.; Muthuchamy, M. MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. Am. J. Physiol Cell Physiol 2015, 309, C680–692. [Google Scholar] [CrossRef] [Green Version]
- Kontarakis, Z.; Rossi, A.; Ramas, S.; Dellinger, M.T.; Stainier, D.Y.R. Mir-126 is a conserved modulator of lymphatic development. Dev. Biol 2018, 437, 120–130. [Google Scholar] [CrossRef]
- Borza, C.M.; Pozzi, A. Discoidin domain receptors in disease. Matrix Biol 2014, 34, 185–192. [Google Scholar] [CrossRef]
- Oh, S.; Seo, M.; Choi, J.S.; Joo, C.K.; Lee, S.K. MiR-199a/b-5p Inhibits Lymphangiogenesis by Targeting Discoidin Domain Receptor 1 in Corneal Injury. Mol. Cells 2018, 41, 93–102. [Google Scholar] [CrossRef]
- Yamanaka, R.; Arao, T.; Yajima, N.; Tsuchiya, N.; Homma, J.; Tanaka, R.; Sano, M.; Oide, A.; Sekijima, M.; Nishio, K. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene 2006, 25, 5994–6002. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Radziejewski, C.; Campbell, E.; Kovac, L.; McGlynn, M.; Ryan, T.E.; Davis, S.; Goldfarb, M.P.; Glass, D.J.; Lemke, G.; et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell 1997, 1, 25–34. [Google Scholar] [CrossRef]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Xiao, Q.; Jiang, Y.; Liu, Q.; Yue, J.; Liu, C.; Zhao, X.; Qiao, Y.; Ji, H.; Chen, J.; Ge, G. Minor Type IV Collagen alpha5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1. PLoS Genet. 2015, 11, e1005249. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.P.; Mattick, J.S. Noncoding RNA in development. Mamm Genome 2008, 19, 454–492. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding RNAs: Regulators of disease. J. Pathol 2010, 220, 126–139. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R.; Feng, F.Y.; Chinnaiyan, A.M. The bright side of dark matter: lncRNAs in cancer. J. Clin. Invest. 2016, 126, 2775–2782. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yang, S.; Zhou, Z.; Zhao, X.; Zhong, J.; Reinach, P.S.; Yan, D. The Long Noncoding RNA Landscape of the Mouse Eye. Invest. Ophthalmol Vis. Sci 2017, 58, 6308–6317. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Ding, Y.; Jin, X.; Xu, S.; Zhang, H. Long non-coding RNA H19 promotes corneal neovascularization by targeting microRNA-29c. Biosci Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ou, C.; Ren, W.; Xie, X.; Li, X.; Li, G. Downregulation of long non-coding RNA ANRIL suppresses lymphangiogenesis and lymphatic metastasis in colorectal cancer. Oncotarget 2016, 7, 47536–47555. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Zhong, G.; Jiang, N.; Wang, B.; Fan, X.; Chen, C.; Chen, X.; Huang, J.; Lin, T. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J. Clin. Invest. 2018, 128, 861–875. [Google Scholar] [CrossRef] [Green Version]
- Rohan, R.M.; Fernandez, A.; Udagawa, T.; Yuan, J.; D’Amato, R.J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000, 14, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.S.; Rohan, R.M.; Birsner, A.E.; D’Amato, R.J. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. FASEB J. 2003, 17, 2112–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.S.; Rohan, R.M.; Birsner, A.E.; D’Amato, R.J. Genetic loci that control the angiogenic response to basic fibroblast growth factor. FASEB J. 2004, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Regenfuss, B.; Onderka, J.; Bock, F.; Hos, D.; Maruyama, K.; Cursiefen, C. Genetic heterogeneity of lymphangiogenesis in different mouse strains. Am. J. Pathol 2010, 177, 501–510. [Google Scholar] [CrossRef]
- Regenfuss, B.; Dreisow, M.L.; Hos, D.; Masli, S.; Bock, F.; Cursiefen, C. The Naive Murine Cornea as a Model System to Identify Novel Endogenous Regulators of Lymphangiogenesis: TRAIL and rtPA. Lymphat Res. Biol 2015, 13, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Bock, F.; Onderka, J.; Hos, D.; Horn, F.; Martus, P.; Cursiefen, C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp. Eye Res. 2008, 87, 462–470. [Google Scholar] [CrossRef]
- Buttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase Is a Novel Endogenous Regulator of Developmental and Inflammatory Lymphangiogenesis. Am. J. Pathol 2019, 189, 440–448. [Google Scholar] [CrossRef] [Green Version]



| Proteins in Lymphangiogenesis | |||
|---|---|---|---|
| Protein | Function | ||
| Endostatin | Endostatin | inhibition of angiogenesis | [32,37,38,39] |
| Tumstatin | inhibition of angiogenesis | [40,41] | |
| Arrestin | inhibition of angiogenesis | [41] | |
| Plasminogen | Angiostatin | inhibition angiogenesis | [42,43] |
| Thrombospondin | TSP-1 | inhibition of angiogenesis and lymphangiogenesis | [44,83] |
| TSP-2 | inhibition of angiogenesis | [31] | |
| soluble VEGFR | sVEGFR-1 | decoy receptor for VEGF-A; inhibition of angiogenesis | [28,46] |
| sVEGFR-2 | decoy receptor for VEGF-C and -D; inhibition of lymphangiogenesis | [47] | |
| sVEGFR-3 | decoy receptor for VEGF-C and -D; inhibition of lymphangiogenesis | [48] | |
| adapter protein | IRS-1 | treatment with antisense oligonucleotide inhibits hem- and lymphangiogenesis | [65] |
| Glycoprotein | Podoplanin | implication in lymphocyte trafficking, blocking antibody inhibits lymphangiogenesis | [71,72] |
| Integrine | Integrin α5β1 | treatment with antagonist JSM6227 inhibits lymphangiogenesis | [3] |
| Integrin α9β1 | blocking antibody improves graft survival | [75] | |
| Semaphorine | Semaphorin-3F | contributing to anti-(lymph) angiogenic barrier | [82] |
| Vasohibin | VASH-1 | negative feedback; regulator inhibition of angiogenesis and lymphangiogenesis | [84,86] |
| transmembrane Receptor | Neuropilin-2 | associated with VEGFR-3, artificial miRNA improves graft | [87] |
| Metalloproteases | MT-MMP1 | cleavage of VEGFR-1 and LYVE-1 | [91,94] |
| MMP-2 & MMP9 | blockade with SB-3CT inhibits lymphangiogenesis | [95] | |
| Peptide hormone | VIP | inhibition of lymphangiogenesis | [96] |
| α-MSH | inhibition of lymphangiogenesis | [96] | |
| TNF/TNFR-Superfamily | Trail | inhibition of lymphangiogenesis | [135] |
| Proteases | tPA | inhibition of lymphangiogenesis | [135] |
| Membrane protein | Tyrosinase | inhibition of lymphangiogenesis | [137] |
| ncRNAs in Lymphangiogenesis | |||
| Targets | Function | ||
| miRNA-184 | LECs | suppresses migration and adhesion | [104] |
| miRNA-181a | Prox-1 | degradation of Prox-1 | [108] |
| miRNA-31 | Prox-1 | degradation of Prox-1 | [109] |
| miRNA-466 | Prox-1 | degradation of Prox-1 | [110] |
| miRNA-1236 | VEGFR-3 | inhibition of VEGFR-3 | [114] |
| miRNA-9 | VEGFR-3 | increased VEGFR-3 expression | [115] |
| miRNA-126 | VEGFR-2/VEGFR-3 | modulates VEGFR-2 and VEGFR-3 signal transduction | [116] |
| miRNA-199a/b5p | DDR1 | degradation of DDR1 | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clahsen, T.; Büttner, C.; Hatami, N.; Reis, A.; Cursiefen, C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. J. Clin. Med. 2020, 9, 479. https://doi.org/10.3390/jcm9020479
Clahsen T, Büttner C, Hatami N, Reis A, Cursiefen C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. Journal of Clinical Medicine. 2020; 9(2):479. https://doi.org/10.3390/jcm9020479
Chicago/Turabian StyleClahsen, Thomas, Christian Büttner, Niloofar Hatami, André Reis, and Claus Cursiefen. 2020. "Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation" Journal of Clinical Medicine 9, no. 2: 479. https://doi.org/10.3390/jcm9020479
APA StyleClahsen, T., Büttner, C., Hatami, N., Reis, A., & Cursiefen, C. (2020). Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. Journal of Clinical Medicine, 9(2), 479. https://doi.org/10.3390/jcm9020479





