Photodynamic Therapy in Primary Breast Cancer
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Patient Selection
- Full medical history, including last menstrual period (LMP).
- Current medication.
- Pregnancy test if not postmenopausal or menstruating.
- Pre-treatment MRI scan of both breasts using dedicated breast coils, with and without contrast enhancement, if not done as a part of their initial diagnostic pathway.
- Routine blood investigations: blood count, urea and electrolytes, liver function tests.
2.3. Photodynamic Therapy
3. Results
3.1. Quantification of PDT Effects
3.2. Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cengel, K.A.; Simone II, C.B.; Glatstein, E. PDT: What’s Past Is Prologue. Cancer Res. 2016, 76, 2497–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bown, S.G. Photodynamic therapy for photochemists. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120371. [Google Scholar] [CrossRef] [PubMed]
- Kleinovink, J.W.; Fransen, M.F.; Lowik, C.W.; Ossendorp, F. Photodynamic-Immune Checkpoint Therapy Eradicates Local and Distant Tumors by CD8(+) T Cells. Cancer Immunol. Res. 2017, 5, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampa, M.; Sarbu, M.I.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Photodynamic therapy: A hot topic in dermato-oncology. Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Vander Poorten, V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, T.; Zhang, Y.; Dang, G. Anti-VEGF Monotherapy Versus Photodynamic Therapy and Anti-VEGF Combination Treatment for Neovascular Age-Related Macular Degeneration: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4307–4317. [Google Scholar] [CrossRef] [Green Version]
- Lou, P.J.; Jager, H.R.; Jones, L.; Theodossy, T.; Bown, S.G.; Hopper, C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer 2004, 91, 441–446. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef] [Green Version]
- Azzouzi, A.R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet. Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
- A Summary of the European Public Assessment Report (EPAR) for Tookad. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tookad (accessed on 17 August 2019).
- Morrison, S.A.; Hill, S.L.; Rogers, G.S.; Graham, R.A. Efficacy and safety of continuous low-irradiance photodynamic therapy in the treatment of chest wall progression of breast cancer. J. Surg. Res. 2014, 192, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; Keshtgar, M.R. Electrochemotherapy for Treatment of Cutaneous Breast Cancer Metastases: A Review. Arch. Breast Cancer 2016, 3, 108–117. [Google Scholar]
- Kessel, D. Apoptosis and associated phenomena as a determinants of the efficacy of photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMichele, A.; Yee, D.; Esserman, L. Mechanisms of Resistance to Neoadjuvant Chemotherapy in Breast Cancer. N. Engl. J. Med. 2017, 377, 2287–2289. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, M.; Davis, S.C.; Dehghani, H.; Huggett, M.T.; Hasan, T.; Pereira, S.P.; Bown, S.G.; Pogue, B.W. CT contrast predicts pancreatic cancer treatment response to verteporfin-based photodynamic therapy. Phys. Med. Biol. 2014, 59, 1911–1921. [Google Scholar] [CrossRef] [Green Version]
- Weijer, R.; Broekgaarden, M.; Krekorian, M.; Alles, L.K.; van Wijk, A.C.; Mackaaij, C.; Verheij, J.; van der Wal, A.C.; van Gulik, T.M.; Storm, G.; et al. Inhibition of hypoxia inducible factor 1 and topoisomerase with acriflavine sensitizes perihilar cholangiocarcinomas to photodynamic therapy. Oncotarget 2016, 7, 3341–3356. [Google Scholar] [CrossRef] [Green Version]
- Olsen, C.E.; Weyergang, A.; Edwards, V.T.; Berg, K.; Brech, A.; Weisheit, S.; Hogset, A.; Selbo, P.K. Development of resistance to photodynamic therapy (PDT) in human breast cancer cells is photosensitizer-dependent: Possible mechanisms and approaches for overcoming PDT-resistance. Biochem. Pharmacol. 2017, 144, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Bedwell, J.; MacRobert, A.J.; Phillips, D.; Bown, S.G. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br. J. Cancer 1992, 65, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Barr, H.; Tralau, C.J.; Boulos, P.B.; MacRobert, A.J.; Tilly, R.; Bown, S.G. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem. Photobiol. 1987, 46, 795–800. [Google Scholar] [CrossRef]
- Chang, S.C.; Buonaccorsi, G.; MacRobert, A.; Bown, S.G. Interstitial and transurethral photodynamic therapy of the canine prostate using meso-tetra-(m-hydroxyphenyl) chlorin. Int. J. Cancer 1996, 67, 555–562. [Google Scholar] [CrossRef]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A.; Hatfield, A.W. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, H.; Hall-Craggs, M.A.; Wotherspoon, A.; Paley, M.; Buonaccorsi, G.; Amin, Z.; Wilkinson, I.; Kissin, M.W.; Davidson, T.I.; Taylor, I.; et al. Laser therapy for breast cancer: MR imaging and histopathologic correlation. Radiology 1996, 200, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. RFA experiences, indications and clinical outcomes. Int. J. Clin. Oncol. 2019, 24, 603–607. [Google Scholar] [CrossRef]
- Mauri, G.; Sconfienza, L.M.; Pescatori, L.C.; Fedeli, M.P.; Ali, M.; Di Leo, G.; Sardanelli, F. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 3199–3210. [Google Scholar] [CrossRef]
- Takada, M.; Toi, M. Cryosurgery for primary breast cancers, its biological impact, and clinical outcomes. Int. J. Clin. Oncol. 2019, 24, 608–613. [Google Scholar] [CrossRef]
- Peek, M.C.L.; Wu, F. High-intensity focused ultrasound in the treatment of breast tumours. Ecancermedicalscience 2018, 12, 794. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Liu, C.; Zeng, Y.; Hao, Y.; Huang, J.; Yang, Z.; Li, R. Nanoceria-mediated drug delivery for targeted photodynamic therapy on drug-resistant breast cancer. ACS Appl. Mater. Interfaces 2016, 8, 31510–31523. [Google Scholar]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Phthalocyanine induced phototherapy coupled with Doxorubicin; a promising novel treatment for breast cancer. Expert Rev. AntiCancer Ther. 2017, 17, 693–702. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Women age 30 years or over | 1. Ductal carcinoma in situ (DCIS) without invasive carcinoma |
| 2. Confirmed invasive ductal carcinoma (IDC) | 2. Invasive lobular carcinoma |
| 3. Unifocal tumor or unifocal site deemed suitable for PDT (Photodynamic Therapy) in multifocal invasive ductal carcinoma in a single breast | 3. Current participation in any other trial of experimental medicine, or on current endocrine medication or neo-adjuvant therapy |
| 4. Scheduled for surgery + axillary staging as primary treatment | 4. Known metastatic disease |
| 5. Negative pregnancy test within 7 days of registration for trial | 5. Pregnancy and lactation |
| 6. Willing to use contraception from date of consent until 6 weeks after completion of treatment | 6. Severe cardiovascular or other systemic disease |
| 7. Not breastfeeding | 7. Known porphyria or sensitivity to photosensitizers |
| 8. Capable of giving written informed consent | 8. Any mental disorder making reliable informed consent impossible |
| Characteristics | PDT Cohort |
|---|---|
| Total | 12 |
| Median age | 49 (30–79) |
| Menopausal status | 3 post-menopausal; 9 pre-menopausal |
| Estrogen (ER), Progesterone (PR) and Her 2 Receptor status: Group 1: ER + ve, PR + ve, Her 2 − ve | 10 |
| Group 2: ER − ve, PR − ve, Her 2 − ve | 1 |
| Group 3: ER + ve, PR + ve, Her 2 + ve | 1 |
| Tumor size | T2 = 4; T3 or greater = 8 |
| Grade | G2 = 5, G3 = 7 |
| Nodal status at mastectomy | All patients had positive nodes |
| Distant metastases at presentation | None |
| Primary treatment (after PDT) | Mastectomy |
| Adjuvant radiotherapy after PDT and mastectomy | 12 |
| Adjuvant chemotherapy after PDT and mastectomy | 12 |
| Adjuvant endocrine therapy after PDT and mastectomy | 9 |
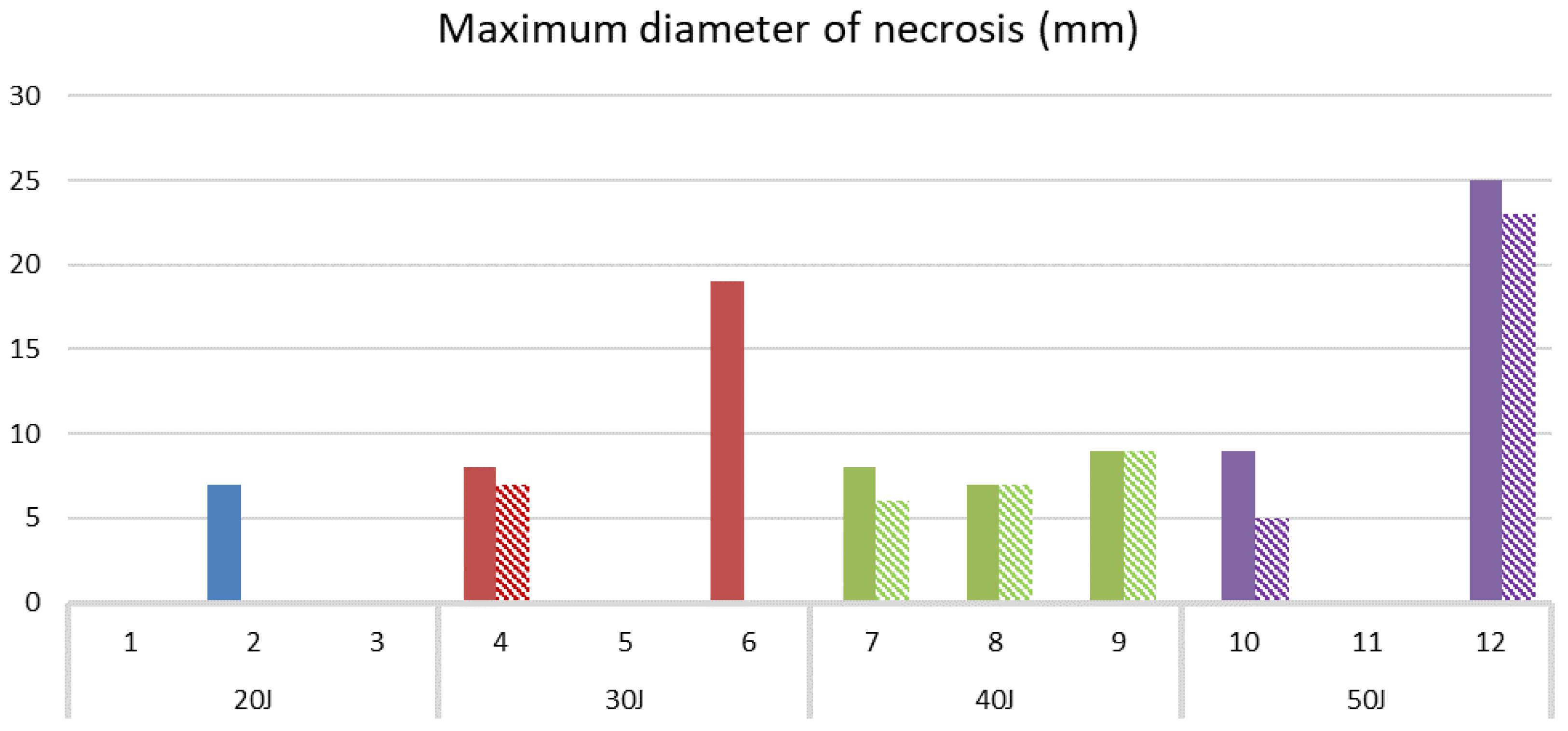
| Pt | Age | MRI Total Tumor Vol (mm3) | Pre-PDT Necrosis (mm3) | PDT Dose (J) | PDT Necrosis on MRI (mm3) | PDT Necrosis on Histology (mm3) | Depth of Needle Tip (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 79 | 21,800 | 170 | 20 | 0 | 0 | n/a |
| 2 | 45 | 91,100 | 1400 | 20 | 0 | 180 | 5 |
| 3 | 53 | n/a | n/a | 20 | n/a | 0 | n/a |
| 4 | 57 | 32,800 | <10 | 30 | 180 | 65 | 6 |
| 5 | 36 | 198,000 | 0 | 30 | 0 | 0 | n/a |
| 6 | 49 | 433,000 | 2600 | 30 | # | 5576 | 12 |
| 7 | 30 | 135,000 | 0 | 40 | 78 | 270 | 10 |
| 8 | 48 | 235,000 | 0 | 40 | 180 | 180 | 13 |
| 9 | 47 | 3700 | 0 | 40 | 109 | 380 | n/a |
| 10 | 57 | 27,800 | 0 | 50 | 253 | 380 | 10 |
| 11 | 54 | 27,500 | 0 | 50 | 0 | 0 | 8 |
| 12 | 49 | 51,400 | 0 | 50 | 8316 | 8182 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. https://doi.org/10.3390/jcm9020483
Banerjee SM, El-Sheikh S, Malhotra A, Mosse CA, Parker S, Williams NR, MacRobert AJ, Hamoudi R, Bown SG, Keshtgar MRS. Photodynamic Therapy in Primary Breast Cancer. Journal of Clinical Medicine. 2020; 9(2):483. https://doi.org/10.3390/jcm9020483
Chicago/Turabian StyleBanerjee, Shramana M., Soha El-Sheikh, Anmol Malhotra, Charles A. Mosse, Sweta Parker, Norman R. Williams, Alexander J. MacRobert, Rifat Hamoudi, Stephen G. Bown, and Mo R. S. Keshtgar. 2020. "Photodynamic Therapy in Primary Breast Cancer" Journal of Clinical Medicine 9, no. 2: 483. https://doi.org/10.3390/jcm9020483
APA StyleBanerjee, S. M., El-Sheikh, S., Malhotra, A., Mosse, C. A., Parker, S., Williams, N. R., MacRobert, A. J., Hamoudi, R., Bown, S. G., & Keshtgar, M. R. S. (2020). Photodynamic Therapy in Primary Breast Cancer. Journal of Clinical Medicine, 9(2), 483. https://doi.org/10.3390/jcm9020483





