Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Pulp Cells
2.2. Exosome Isolation
2.3. Negative Staining for Transmission Electron Microscopy (TEM)
2.4. Western Blot
2.5. Human Bone Marrow-Derived Mesenchymal Stem Cells (HBMMSCs)
2.6. Uptake of Exosomes by HBMMSCs
2.7. Fibrin Gel Preparation
2.8. Cell Migration
2.9. Cell Proliferation
- DMEM + exosomes (10 µL)
- DMEM + 10% FBS
- DMEM + 10% FBS + exosomes (10 µL)
- DMEM + 20% FBS
2.10. Statistical Analysis
3. Results
3.1. Characterization of Exosomes Derived from Dental Pulp
3.2. Uptake of Labeled Exosomes by HBMMSCs
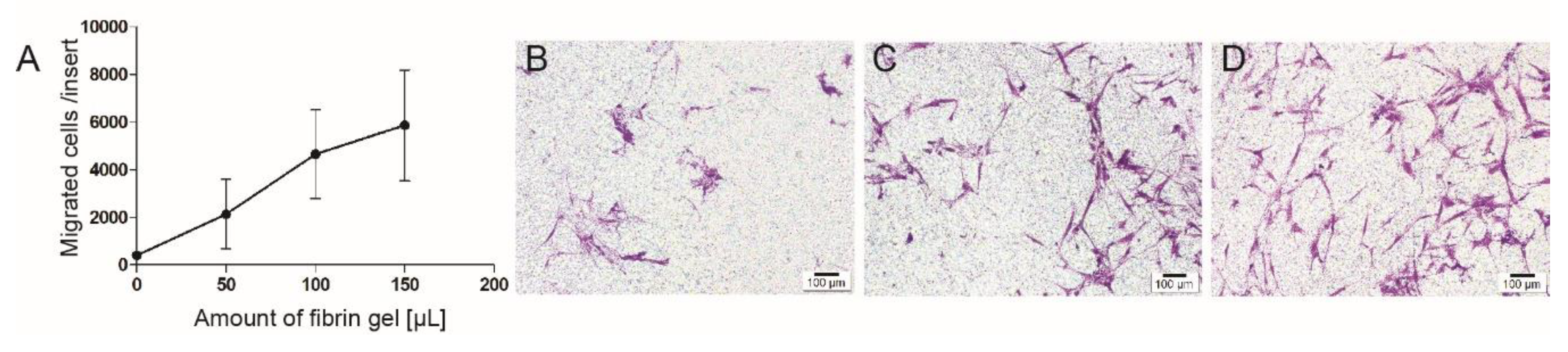
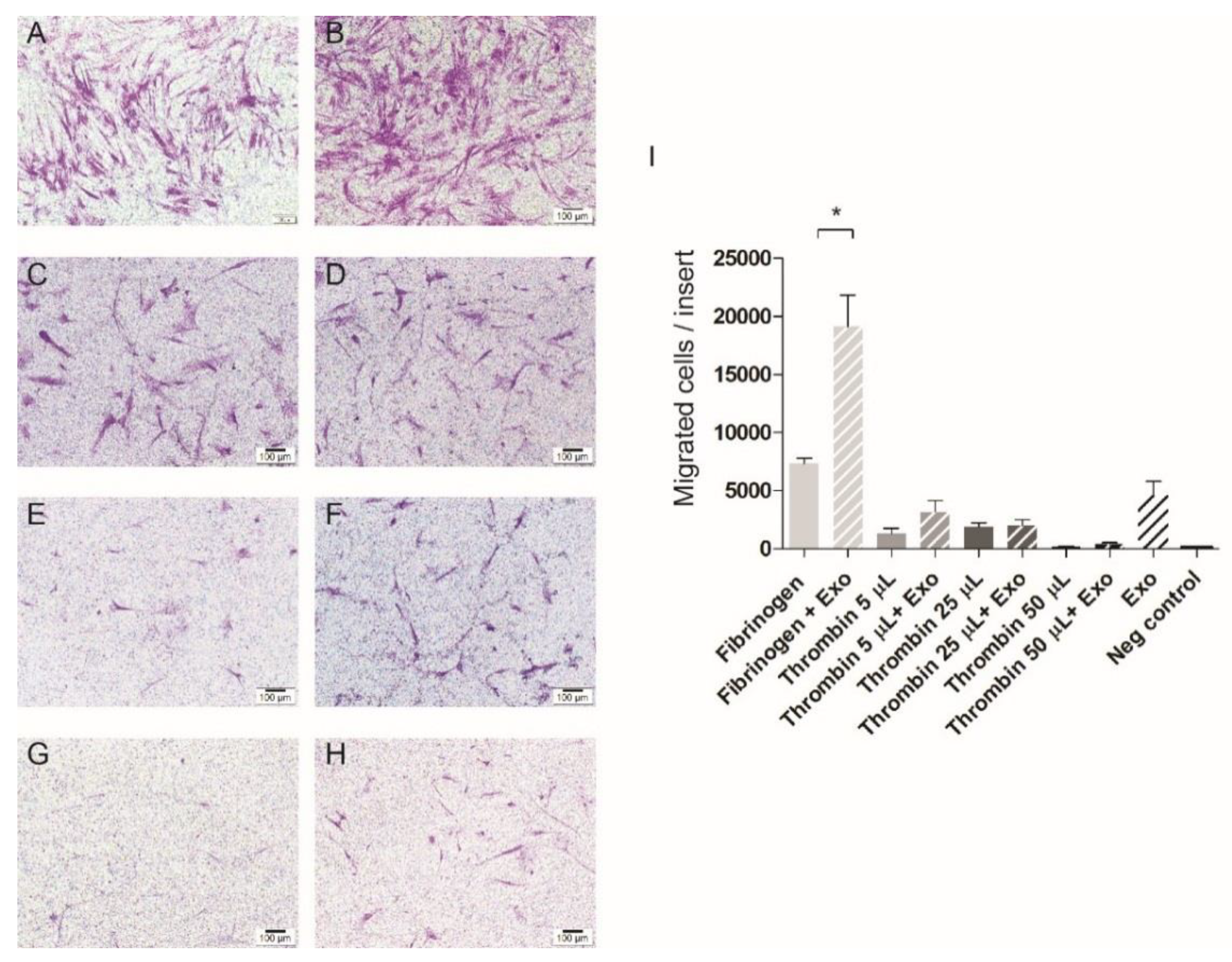
3.3. Cell Migration
3.4. Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European society of endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists. Scope of Endodontics: Regenerative Endodontics. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/scopeofendo_regendo.pdf (accessed on 2 November 2019).
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M. Clinical procedures for revitalization: Current knowledge and considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef]
- Nosrat, A.; Homayounfar, N.; Oloomi, K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: A literature review and report of a case. J. Endod. 2012, 38, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Tremain, N.; Korkko, J.; Ibberson, D.; Kopen, G.C.; DiGirolamo, C.; Phinney, D.G. Microsage analysis of 2353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mrnas of multiple cell lineages. Stem Cells 2001, 19, 408–418. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of cd105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Mao, J.J.; Kim, S.G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative endodontics: Barriers and strategies for clinical translation. Dent. Clin. N. Am. 2012, 56, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Ruangsawasdi, N.; Zehnder, M.; Patcas, R.; Ghayor, C.; Siegenthaler, B.; Gjoksi, B.; Weber, F.E. Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Eng. Part A 2017, 23, 115–123. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Y.F.; Sun, Z.Y.; Song, G.T.; Chen, Z. Dental pulp tissue engineering with bfgf-incorporated silk fibroin scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Damania, A.; Jaiman, D.; Teotia, A.K.; Kumar, A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res. Ther. 2018, 9, 31. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Borger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef]
- Sze, S.K.; de Kleijn, D.P.; Lai, R.C.; Khia Way Tan, E.; Zhao, H.; Yeo, K.S.; Low, T.Y.; Lian, Q.; Lee, C.N.; Mitchell, W.; et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol. Cell. Proteom. MCP 2007, 6, 1680–1689. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Ruangsawasdi, N.; Zehnder, M.; Weber, F.E. Fibrin gel improves tissue ingrowth and cell differentiation in human immature premolars implanted in rats. J. Endod. 2014, 40, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Hertig, G.; Zehnder, M.; Woloszyk, A.; Mitsiadis, T.A.; Ivica, A.; Weber, F.E. Iodixanol as a contrast agent in a fibrin hydrogel for endodontic applications. Front. Physiol. 2017, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Gopferich, A.; Schmalz, G. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Francis, C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000, 96, 3772–3778. [Google Scholar] [CrossRef]
- Hao, C.; Wang, Y.; Shao, L.; Liu, J.; Chen, L.; Zhao, Z. Local injection of bone mesenchymal stem cells and fibrin glue promotes the repair of bone atrophic nonunion in vivo. Adv. Ther. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Blazquez, R.; Sanchez-Margallo, F.M.; Alvarez, V.; Uson, A.; Marinaro, F.; Casado, J.G. Fibrin glue mesh fixation combined with mesenchymal stem cells or exosomes modulates the inflammatory reaction in a murine model of incisional hernia. Acta Biomater. 2018, 71, 318–329. [Google Scholar] [CrossRef]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of apical mesenchymal stem cells into root canals of mature teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef]
- European Medicines Agency. Fibrinogen-Containing Solutions. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/fibrinogen-containing-solutions-sealant-authorised-administration-spray-application (accessed on 8 November 2019).
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Jiang, X.C.; Gao, J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp stem cell-mediated functional pulp regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Yang, F.H.; Wang, Y.J.; Pei, F.; Chen, Z.; Zhang, L. Odontoblastic exosomes attenuate apoptosis in neighboring cells. J. Dent. Res. 2019, 98, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- About, I. Dentin regeneration in vitro: The pivotal role of supportive cells. Adv. Dent. Res. 2011, 23, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; About, I.; Chung, S.H. Pulp fibroblasts control nerve regeneration through complement activation. J. Dent. Res. 2016, 95, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, M.; Matsuzaka, K.; Kokubu, E.; Azuma, T.; Inoue, T. Analysis of side population cells derived from dental pulp tissue. Int. Endod. J. 2010, 43, 1132–1142. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sanchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Sharma, S.; Alharbi, M.; Kobayashi, M.; Lai, A.; Guanzon, D.; Zuniga, F.; Ormazabal, V.; Palma, C.; Scholz-Romero, K.; Rice, G.E.; et al. Proteomic analysis of exosomes reveals an association between cell invasiveness and exosomal bioactivity on endothelial and mesenchymal cell migration in vitro. Clin. Sci. 2018, 132, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Baxa, U. Imaging of liposomes by transmission electron microscopy. Methods Mol. Biol. 2018, 1682, 73–88. [Google Scholar]
- Nosrat, A.; Ryul Kim, J.; Verma, P.; Chand, P.S. Tissue engineering considerations in dental pulp regeneration. Iran. Endod. J. 2014, 9, 30–39. [Google Scholar] [PubMed]
- Naito, M. Effects of fibrinogen, fibrin and their degradation products on the behaviour of vascular smooth muscle cells. Nihon Ronen Igakkai Zasshi. Jpn. J. Geriatr. 2000, 37, 458–463. [Google Scholar] [CrossRef][Green Version]
- Seeger, F.H.; Blessing, E.; Gu, L.; Bornhold, R.; Denger, S.; Kreuzer, J. Fibrinogen induces chemotactic activity in endothelial cells. Acta Physiol. Scand. 2002, 176, 109–115. [Google Scholar] [CrossRef]
- Bar-Shavit, R.; Kahn, A.; Fenton, J.W., II; Wilner, G.D. Chemotactic response of monocytes to thrombin. J. Cell Biol. 1983, 96, 282–285. [Google Scholar] [CrossRef]
- Catelas, I.; Sese, N.; Wu, B.M.; Dunn, J.C.; Helgerson, S.; Tawil, B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006, 12, 2385–2396. [Google Scholar] [CrossRef]
- Sirieix, D.; Chemla, E.; Castier, Y.; Massonnet-Castel, S.; Fabiani, J.N.; Baron, J.F. Comparative study of different biological glues in an experimental model of surgical bleeding in anesthetized rats: Platelet-rich and -poor plasma-based glue with and without aprotinin versus commercial fibrinogen-based glue. Ann. Vasc. Surg. 1998, 12, 311–316. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivica, A.; Ghayor, C.; Zehnder, M.; Valdec, S.; Weber, F.E. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. J. Clin. Med. 2020, 9, 491. https://doi.org/10.3390/jcm9020491
Ivica A, Ghayor C, Zehnder M, Valdec S, Weber FE. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. Journal of Clinical Medicine. 2020; 9(2):491. https://doi.org/10.3390/jcm9020491
Chicago/Turabian StyleIvica, Anja, Chafik Ghayor, Matthias Zehnder, Silvio Valdec, and Franz E. Weber. 2020. "Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material" Journal of Clinical Medicine 9, no. 2: 491. https://doi.org/10.3390/jcm9020491
APA StyleIvica, A., Ghayor, C., Zehnder, M., Valdec, S., & Weber, F. E. (2020). Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. Journal of Clinical Medicine, 9(2), 491. https://doi.org/10.3390/jcm9020491





