Risk Factors for Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: What Is the Influence of XDR Phenotype on Outcomes?
Abstract
:1. Introduction
2. Methods
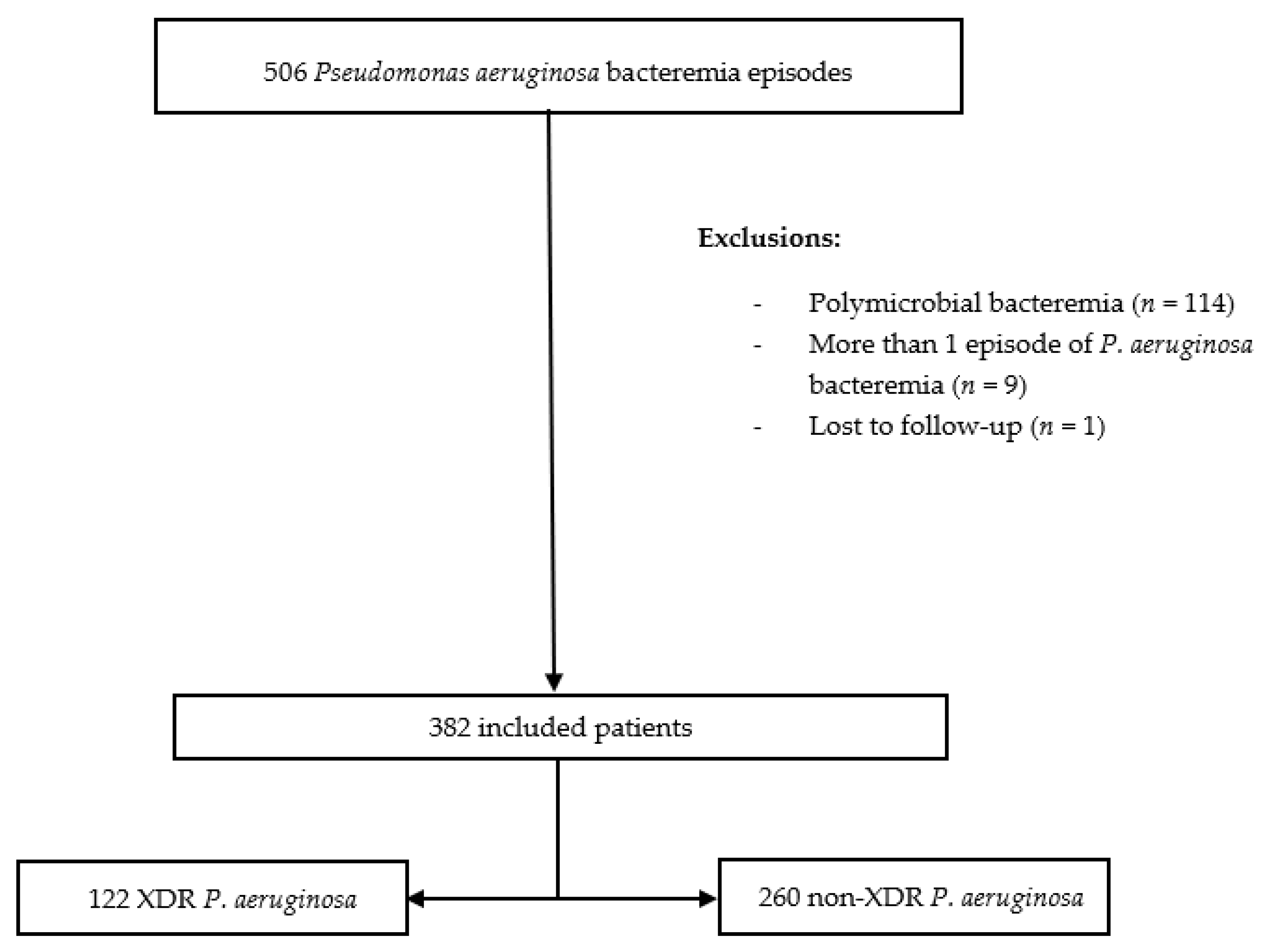
2.1. Hospital Setting, Study Design, and Participants
2.2. Study Objectives and Outcomes
2.3. Variables and Data Source
2.4. Microbiological Studies
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Mortality and Risk Factors for Mortality
3.2. Subanalysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates 2015, 21–22, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabot, G.; Ocampo-Sosa, A.A.; Domínguez, M.A.; Gago, J.F.; Juan, C.; Tubau, F.; Rodríguez, C.; Moyà, B.; Peña, C.; Martínez-Martínez, L.; et al. Genetic Markers of Widespread Extensively Drug-Resistant Pseudomonas aeruginosa High-Risk Clones. Antimicrob. Agents Chemother. 2012, 56, 6349–6357. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Kollef, M.H. Broad-Spectrum Antimicrobials and the Treatment of Serious Bacterial Infections: Getting It Right Up Front. Clin. Infect. Dis. 2008, 47, S3–S13. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef] [Green Version]
- Vidal, F.; Mensa, J.; Almela, M.; Martinez, J.A.; Marco, F.; Casals, C.; Gatell, J.M.; Soriano, E.; de Jimenez Anta, M.T. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 1996, 156, 2121–2126. [Google Scholar] [CrossRef]
- Peña, C.; Suarez, C.; Gozalo, M.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; Calbo, E.; Rodríguez-Baño, J.; et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob. Agents Chemother. 2012, 56, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Kim, S.; Kim, H.; Park, S.; Choe, Y.; Oh, M.; Kim, E.; Choe, K. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.L.; Dantas, R.C.; Ribas, R.M.; Gontijo-Filho, P.P. Pseudomonas aeruginosa bacteraemia: Independent risk factors for mortality and impact of resistance on outcome. J. Med. Microbiol. 2014, 63, 1679–1687. [Google Scholar]
- Suárez, C.; Peña, C.; Gavaldà, L.; Tubau, F.; Manzur, A.; Dominguez, M.A.; Pujol, M.; Gudiol, F.; Ariza, J. Influence of carbapenem resistance on mortality and the dynamics of mortality in Pseudomonas aeruginosa bloodstream infection. Int. J. Infect. Dis. 2010, 14, e73–e78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, E.-J.; Kang, C.-I.; Ha, Y.E.; Kang, S.-J.; Park, S.Y.; Chung, D.R.; Peck, K.R.; Lee, N.Y.; Song, J.-H. Risk Factors for Mortality in Patients with Pseudomonas aeruginosa Bacteremia: Clinical Impact of Antimicrobial Resistance on Outcome. Microb. Drug Resist. 2011, 17, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Rogers, C.A.; Chang, K.T.; Weston, J.S.; Caeiro, J.P.; Garey, K.W. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob. Agents Chemother. 2010, 54, 3717–3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteremia—Retrospective multicenter study. Int. J. Antimicrob. Agents 2019, 55, 105847. [Google Scholar] [CrossRef]
- Recio, R.; Mancheño, M.; Viedma, E.; Villa, J.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Predictors of Mortality in Bloodstream Infections Caused by Pseudomonas aeruginosa: Impact of Antimicrobial Resistance and Bacterial Virulence. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- Peña, C.; Cabot, G.; Gómez-Zorrilla, S.; Zamorano, L.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; et al. Influence of virulence genotype and resistance profile in the mortality of Pseudomonas aeruginosa bloodstream infections. Clin. Infect. Dis. 2015, 60, 539–548. [Google Scholar] [CrossRef]
- Del Barrio-Tofiño, E.; López-Causapé, C.; Cabot, G.; Rivera, A.; Benito, N.; Segura, C.; Montero, M.M.; Sorlí, L.; Tubau, F.; Gómez-Zorrilla, S.; et al. Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob. Agents Chemother. 2017, 61, e01589-17. [Google Scholar] [CrossRef] [Green Version]
- del Barrio-Tofiño, E.; Zamorano, L.; Cortes-Lara, S.; López-Causapé, C.; Sánchez-Diener, I.; Cabot, G.; Bou, G.; Martínez-Martínez, L.; Oliver, A.; Group, G.-S.P. Study Spanish nationwide survey on Pseudomonas aeruginosa antimicrobial resistance mechanisms and epidemiology. J. Antimicrob. Chemother. 2019, 74, 1825–1835. [Google Scholar] [CrossRef]
- Montero, M.; VanScoy, B.D.; López-Causapé, C.; Conde, H.; Adams, J.; Segura, C.; Zamorano, L.; Oliver, A.; Horcajada, J.P.; Ambrose, P.G. Evaluation of ceftolozane-tazobactam in combination with meropenem against Pseudomonas aeruginosa sequence type 175 in a hollow-fiber infection model. Antimicrob. Agents Chemother. 2018, 62, e00026-18. [Google Scholar] [CrossRef] [Green Version]
- Montero, M.M.; Domene Ochoa, S.; López-Causapé, C.; VanScoy, B.; Luque, S.; Sorlí, L.; Campillo, N.; Padilla, E.; Prim, N.; Segura, C.; et al. Colistin plus meropenem combination is synergistic in vitro against extensively drug-resistant Pseudomonas aeruginosa, including high-risk clones. J. Glob. Antimicrob. Resist. 2019, 18, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- McCabe WR, J.G. Gram negative bacteremia: I. Etiology and ecology. Arch. Intern. Med. 1962, 110, 845–847. [Google Scholar] [CrossRef]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; Mcgarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; Macfarquhar, J.; Walton, A.L.; et al. Health Care—Associated Bloodstream Infections in Adults: A Reason To Change the Accepted Definition of Community—Acquired Infections. Ann. Fam. Med. 2002, 137, 791–798. [Google Scholar] [CrossRef]
- Rhee, J.Y.; Kwon, K.T.; Ki, H.K.; Shin, S.Y.; Jung, D.S.; Chung, D.R.; Ha, B.C.; Peck, K.R.; Song, J.H. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: A comparison of the PITT bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock 2009, 31, 146–150. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe—Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017; ECDC: Stockholm, Sweden, 2018; ISBN 9789294982797. [Google Scholar]
- Shortridge, D.; Gales, A.C.; Streit, J.M.; Huband, M.D.; Tsakris, A.; Jones, R.N. Geographic and Temporal Patterns of Antimicrobial Resistance in Pseudomonas aeruginosa Over 20 Years From the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6, S63–S68. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Shields, R.K.; Clarke, L.G.; Potoski, B.A.; Clancy, C.J.; Hong Nguyen, M. Carbapenem-resistant Pseudomonas aeruginosa bacteremia: Risk factors for mortality and microbiologic treatment failure. Antimicrob. Agents Chemother. 2017, 61, e01243-16. [Google Scholar] [CrossRef] [Green Version]
- Recio, R.; Villa, J.; Viedma, E.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Bacteraemia due to extensively drug-resistant Pseudomonas aeruginosa sequence type 235 high-risk clone: Facing the perfect storm. Int. J. Antimicrob. Agents 2018, 52, 172–179. [Google Scholar] [CrossRef]
- Sánchez-Diener, I.; Zamorano, L.; Peña, C.; Ocampo-Sosa, A.; Cabot, G.; Gómez-Zorrilla, S.; Almirante, B.; Aguilar, M.; Granados, A.; Calbo, E.; et al. Weighting the impact of virulence on the outcome of Pseudomonas aeruginosa bloodstream infections. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Sabé, N.; Tubau, F.; Lladó, L.; Baliellas, C.; González-Costello, J.; Cruzado, J.M.; Carratalà, J. Extensively drug-resistant Pseudomonas aeruginosa bacteremia in solid organ transplant recipients. Transplantation 2015, 99, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Palavutitotai, N.; Jitmuang, A.; Tongsai, S.; Kiratisin, P.; Angkasekwinai, N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE 2018, 13, e0193431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samonis, G.; Vardakas, K.Z.; Kofteridis, D.P.; Dimopoulou, D.; Andrianaki, A.M.; Chatzinikolaou, I.; Katsanevaki, E.; Maraki, S.; Falagas, M.E. Characteristics, risk factors and outcomes of adult cancer patients with extensively drug-resistant Pseudomonas aeruginosa infections. Infection 2014, 42, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Osih, R.B.; Mcgregor, J.C.; Rich, S.E.; Moore, A.C.; Furuno, J.P.; Perencevich, E.N.; Harris, A.D. Impact of Empiric Antibiotic Therapy on Outcomes in Patients with Pseudomonas aeruginosa Bacteremia. Antimicrob. Agents Chemother. 2007, 51, 839–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodise, T.P.; Patel, N.; Kwa, A.; Graves, J.; Furuno, J.P.; Graffunder, E.; Lomaestro, B.; McGregor, J.C. Predictors of 30-Day Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: Impact of Delayed Appropriate Antibiotic Selection. Antimicrob. Agents Chemother. 2007, 51, 3510–3515. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Sun, Z.; Jiao, X.; Peng, Q.; Jiang, F.; Huang, Y.; Zhang, J.; Yao, F. Antibiotic Resistance in Pseudomonas aeruginosa is Associated with Decreased Fitness. Cell. Physiol. Biochem. 2013, 31, 347–354. [Google Scholar] [CrossRef]
- Gómez-Zorrilla, S.; Juan, C.; Cabot, G.; Camoez, M.; Tubau, F.; Oliver, A.; Dominguez, M.A.; Ariza, J.; Peña, C. Impact of multidrug resistance on the pathogenicity of Pseudomonas aeruginosa: In vitro and in vivo studies. Int. J. Antimicrob. Agents 2016, 47, 368–374. [Google Scholar] [CrossRef]
- Gómez-Zorrilla, S.; Calatayud, L.; Juan, C.; Cabot, G.; Tubau, F.; Oliver, A.; Dominguez, M.A.; Ariza, J.; Peña, C. Understanding the acute inflammatory response to Pseudomonas aeruginosa infection: Differences between susceptible and multidrug-resistant strains in a mouse peritonitis model. Int. J. Antimicrob. Agents 2017, 49, 198–203. [Google Scholar] [CrossRef]
- Yoon, E.-J.; Kim, D.; Lee, H.; Lee, H.S.; Shin, J.H.; Park, Y.S.; Kim, Y.A.; Shin, J.H.; Shin, K.S.; Uh, Y.; et al. Mortality dynamics of Pseudomonas aeruginosa bloodstream infections and the influence of defective OprD on mortality: Prospective observational study. J. Antimicrob. Chemother. 2019, 74, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Olivares Pacheco, J.; Alvarez-Ortega, C.; Alcalde Rico, M.; Martínez, J.L. Metabolic Compensation of Fitness Costs Is a General Outcome for Antibiotic-Resistant Pseudomonas aeruginosa Mutants Overexpressing Efflux Pumps. mBio 2017, 8, e00500-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skurnik, D.; Roux, D.; Cattoir, V.; Danilchanka, O.; Lu, X.; Yoder-Himes, D.R.; Han, K.; Guillard, T.; Jiang, D.; Gaultier, C.; et al. Enhanced in vivo fitness of carbapenem-resistant oprDmutants of Pseudomonas aeruginosa revealed through high-throughput sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 20747–20752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Khawcharoenporn, T.; Chuncharunee, A.; Maluangnon, C.; Taweesakulvashra, T.; Tiamsak, P. Active monotherapy and combination therapy for extensively drug-resistant Pseudomonas aeruginosa pneumonia. Int. J. Antimicrob. Agents 2018, 52, 828–834. [Google Scholar] [CrossRef]
- Rigatto, M.H.; Vieira, F.J.; Antochevis, L.C.; Behle, T.F.; Lopes, N.T.; Zavascki, A.P. Polymyxin B in Combination with Antimicrobials Lacking In Vitro Activity versus Polymyxin B in aMonotherapy in Critically Ill Patients with Acinetobacter baumannii or Pseudomonas aeruginosa Infections. Antimicrob. Agents Chemother. 2015, 59, 6575–6580. [Google Scholar] [CrossRef] [Green Version]
- Apisarnthanarak, A.; Mundy, L.M. Carbapenem-resistant Pseudomonas aeruginosa pneumonia with intermediate minimum inhibitory concentrations to doripenem: Combination therapy with high-dose, 4-h infusion of doripenem plus fosfomycin versus intravenous colistin plus fosfomycin. Int. J. Antimicrob. Agents 2012, 39, 271–272. [Google Scholar] [CrossRef]
- Ribera, A.; Benavent, E.; Lora-Tamayo, J.; Tubau, F.; Pedrero, S.; Cabo, X.; Ariza, J.; Murillo, O. Osteoarticular infection caused by MDR Pseudomonas aeruginosa: The benefits of combination therapy with colistin plus β-lactams. J. Antimicrob. Chemother. 2015, 70, 3357–3365. [Google Scholar]

| Variable | All Episodes (n = 382) | Non-XDR PA (n = 260) | XDR PA (n = 122) | p-Value |
|---|---|---|---|---|
| Demographic information | ||||
| Age in years, m (IQR) | 70.5 (60–78) | 72 (60.25–78) | 69 (59–76) | 0.219 |
| Male sex | 276 (72.3) | 190 (73.1) | 86 (70.5) | 0.599 |
| Nosocomial acquisition | 244 (63.9) | 143 (55) | 101 (82.8) | <0.001 |
| Underlying condition | ||||
| Diabetes mellitus | 94 (24.6) | 63 (24.2) | 31 (25.4) | 0.803 |
| Chronic obstructive pulmonary disease | 82 (21.6) | 53 (20.5) | 29 (23.8) | 0.475 |
| Cirrhosis | 28 (7.3) | 18 (6.9) | 10 (8.2) | 0.656 |
| Hemodialysis | 25 (6.5) | 18 (6.9) | 7 (5.7) | 0.662 |
| Hematologic malignancy | 58 (15.2) | 31 (11.9) | 27 (22.1) | 0.010 |
| Solid tumor malignancy | 144 (37.7) | 102 (39.2) | 42 (34.4) | 0.366 |
| Neutropenia | 70 (18.3) | 51 (19.6) | 19 (15.6) | 0.341 |
| Charlson comorbidity index, m (IQR) | 4 (2–6) | 4 (2–6) | 4 (3–6) | 0.911 |
| McCabe score | ||||
| Non-fatal McCabe | 107 (28) | 73 (28.1) | 34 (27.9) | 0.966 |
| Rapidly fatal McCabe | 117 (30.6) | 86 (33.1) | 31 (25.4) | 0.130 |
| Ultimately fatal McCabe | 158 (41.4) | 101 (38.8) | 57 (46.7) | 0.145 |
| Source of infection | ||||
| Catheter-related bloodstream infection | 39 (10.2) | 19 (7.3) | 20 (16.4) | 0.006 |
| Urinary tract infection | 116 (30.4) | 74 (28.5) | 42 (34.4) | 0.237 |
| Respiratory infection | 95 (24.9) | 67 (25.8) | 28 (23) | 0.552 |
| Skin and soft tissue infection | 19 (5) | 15 (5.8) | 4 (3.3) | 0.297 |
| Intraabdominal infection | 45 (11.8) | 36 (13.8) | 9 (7.4) | 0.067 |
| Primary or Unknown | 62 (16.2) | 45 (17.3) | 17 (13.9) | 0.404 |
| Other | 6 (1.6) | 4 (1.5) | 2 (1.6) | 1 |
| High-risk source | 227 (59.4) | 167 (64.2) | 60 (49.2) | 0.005 |
| Low-risk source | 155 (40.6) | 93 (35.8) | 62 (50.8) | 0.005 |
| Baseline illness severity | ||||
| Pitt score, m (IQR) | 2 (1–3) | 2 (0–3) | 2 (1–4) | 0.017 |
| Pitt score ≥ 2 | 206 (53.9) | 133 (51.2) | 73 (59.8) | 0.112 |
| Septic shock | 103 (27) | 65 (25) | 38 (31.1) | 0.207 |
| ICU admission | 96 (25.1) | 61 (23.5) | 35 (28.7) | 0.272 |
| Antibiotic management | ||||
| Appropriate empirical treatment | 164 (42.9) | 150 (57.7) | 14 (11.5) | <0.001 |
| Appropriate definitive treatment | 332 (86.9) | 227 (87.3) | 105 (86.1) | 0.737 |
| Combined antimicrobial therapy | 150 (39.3) | 87 (33.5) | 63 (51.6) | 0.001 |
| All-cause mortality | ||||
| Day 14 | 89 (23.3) | 59 (22.7) | 30 (24.6) | 0.682 |
| Day 30 | 118 (30.9) | 77 (29.6) | 41 (33.6) | 0.431 |
| Variables | Alive (n = 293) | Death (n = 89) | Crude HR (95% CI) | p-Value | HR Multivariate (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Demographic information | ||||||
| Age in years, m (IQR) | 70 (59.5–78) | 71 (62.5–78) | 1.01 (0.99–1.02) | 0.252 | 1.01 (0.99–1.03) | 0.119 |
| Male sex | 209 (71.3) | 67 (75.3) | 1.17 (0.72–1.89) | 0.518 | 1.12 (0.69–1.82) | 0.645 |
| Nosocomial acquisition | 186 (63.5) | 58 (65.2) | 1.05 (0.69–1.4) | 0.796 | ||
| Underlying condition | ||||||
| Diabetes mellitus | 73 (24.9) | 21 (23.6) | 0.96 (0.59–1.56) | 0.858 | ||
| Chronic obstructive pulmonary disease | 57 (19.6) | 25 (28.1) | 1.49 (0.94–2.37) | 0.09 | ||
| Cirrhosis | 21 (7.2) | 7 (7.9) | 1.09 (0.51–2.37) | 0.814 | ||
| Hemodialysis | 22 (7.5) | 3 (3.4) | 0.47 (0.15–1.48) | 0.196 | ||
| Hematology malignancy | 42 (14.3) | 16 (18) | 1.22 (0.71–2.1) | 0.465 | ||
| Solid tumor malignancy | 114 (38.9) | 30 (33.7) | 0.81 (0.52–1.26) | 0.354 | ||
| Neutropenia | 51 (17.4) | 19 (21.3) | 1.22 (0.73–2.02) | 0.445 | ||
| Charlson comorbidity index, m (IQR) | 4 (2–6) | 4 (2–6) | 0.99 (0.93–1.07) | 0.996 | ||
| McCabe score | 1.16 (0.89–1.51) | 0.249 | ||||
| Non-fatal McCabe | 83 (28.3) | 24 (27) | 0.98 (0.61–1.56) | 0.932 | ||
| Rapidly fatal McCabe | 96 (32.8) | 21 (23.6) | 0.67 (0.41–1.09) | 0.104 | ||
| Ultimately fatal McCabe | 114 (38.9) | 44 (49.4) | 1.41 (0.93–2.14) | 0.105 | ||
| Origin of bacteremia | ||||||
| Catheter-related bloodstream infection | 37 (12.6) | 2 (2.2) | 0.18 (0.05–0.74) | 0.017 | ||
| Urinary tract infection | 103 (35.2) | 13 (14.6) | 0.36 (0.19–0.65) | 0.001 | ||
| Respiratory infection | 60 (20.5) | 35 (39.3) | 2.17 (1.42–3.32) | <0.001 | ||
| Skin and soft tissue infection | 13 (4.4) | 6 (6.7) | 1.42 (0.62–3.25) | 0.408 | ||
| Intraabdominal infection | 35 (11.9) | 10 (11.2) | 0.94 (0.49–1.81) | 0.852 | ||
| Primary or Unknown | 40 (13.7) | 22 (24.7) | 1.84 (1.14–2.98) | 0.013 | ||
| Other | 5 (1.7) | 1 (1.1) | 0.69 (0.09–4.97) | 0.715 | ||
| High-risk source | 153 (52.2) | 74 (83.1) | 3.85 (2.17–6.67) | <0.001 | 3.07 (1.73–5.46) | <0.001 |
| Low-risk source | 140 (47.8) | 15 (16.9) | 0.26 (0.15–0.46)) | <0.001 | ||
| Baseline illness severity | ||||||
| Pitt score, m (IQR) | 1 (0.5–2) | 4 (1–4) | 1.36 (1.24–1.49) | <0.001 | 1.25 (1.12–1.38) | <0.001 |
| Pitt score ≥ 2 | 140 (47.8) | 66 (74.2) | 2.78 (1.73–4.48) | <0.001 | ||
| Septic shock | 63 (21.5) | 40 (44.9) | 2.59 (1.71–3.95) | <0.001 | 1.75 (1.12–2.75) | 0.015 |
| ICU admission | 56 (19.1) | 40 (44.9) | 2.92 (1.92–4.44) | <0.001 | ||
| Antibiotic management | ||||||
| Appropriate empirical treatment | 126 (43) | 38 (42.7) | 0.99 (0.65–1.51) | 0.968 | ||
| Appropriate definitive treatment | 266 (90.8) | 66 (74.2) | 0.35 (0.22–0.57) | <0.001 | 0.39 (0.24–0.62) | <0.001 |
| Combined antimicrobial therapy | 114 (38.9) | 36 (40.4) | 1.02 (0.67–1.88) | 0.545 | ||
| XDR PA BSI | 92 (31.4) | 30 (33.7) | 1.02 (0.68–1.56) | 0.915 | 1.07 (0.68–1.67) | 0.777 |
| Variables | Alive (n = 264) | Death (n = 118) | Crude HR (95% CI) | p-Value | HR Multivariate (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Demographic information | ||||||
| Age in years, m (IQR) | 70 (59–77.75) | 72 (62.75–78) | 1.01 (0.99–1.02) | 0.159 | 1.01 (0.99–1.03) | 0.052 |
| Male sex | 185 (70.1) | 91 (77.1) | 1.32 (0.85–2.04) | 0.208 | 1.25 (0.8–1.95) | 0.321 |
| Nosocomial acquisition | 162 (61.4) | 82 (69.5) | 1.31 (0.88–1.94) | 0.178 | ||
| Underlying condition | ||||||
| Diabetes mellitus | 66 (25) | 28 (23.7) | 0.95 (0.62–1.46) | 0.828 | ||
| Chronic obstructive pulmonary disease | 50 (19) | 32 (27.4) | 1.47 (0.98–2.21) | 0.062 | 1.1 (0.7–1.73) | 0.675 |
| Cirrhosis | 19 (7.2) | 9 (7.6) | 1.06 (0.54–2.08) | 0.876 | ||
| Hemodialysis | 22 (8.3) | 3 (2.5) | 0.34 (0.11–1.06) | 0.064 | 0.35 (1.01–1.15) | 0.083 |
| Hematology malignancy | 38 (14.4) | 20 (16.9) | 1.15 (0.71–1.86) | 0.575 | ||
| Solid tumor malignancy | 102 (38.6) | 42 (35.6) | 0.88 (0.6–1.28) | 0.505 | ||
| Neutropenia | 48 (18.2) | 22 (18.6) | 1.03 (0.65–1.64) | 0.884 | ||
| Charlson comorbidity index, m (IQR) | 4 (2–6) | 4 (2–6) | 1.01 (0.95–1.07) | 0.744 | ||
| McCabe score | 1.2 (0.96–1.51) | 0.116 | ||||
| Non-fatal McCabe | 77 (29.2) | 30 (25.4) | 0.89 (0.59–1.35) | 0.596 | ||
| Rapidly fatal McCabe | 87 (33) | 30 (25.4) | 0.72 (0.48–1.09) | 0.124 | ||
| Ultimately fatal McCabe | 100 (37.9) | 58 (49.2) | 1.43 (0.99–2.05) | 0.005 | 1.29 (0.89–1.89) | 0.178 |
| Origin of bacteremia | ||||||
| Catheter-related bloodstream infection | 34 (12.9) | 5 (4.2) | 0.34 (0.14–0.83) | 0.017 | ||
| Urinary tract infection | 96 (36.4) | 20 (16.9) | 0.41 (0.25–0.66) | <0.001 | ||
| Respiratory infection | 49 (18.6) | 46 (39) | 2.25 (1.55–3.26) | <0.001 | ||
| Skin and soft tissue infection | 12 (4.5) | 7 (5.9) | 1.26 (0.58–2.69) | 0.558 | ||
| Intraabdominal infection | 29 (11) | 16 (13.6) | 1.18 (0.69–1.99) | 0.537 | ||
| Primary or Unknown | 39 (14.8) | 23 (19.5) | 1.38 (0.87–2.17) | 0.165 | ||
| Other | 5 (1.9) | 1 (0.8) | 0.51 (0.07–3.63) | 0.500 | ||
| High-risk source | 134 (50.8) | 93 (78.8) | 3.03 (1.96–4.76) | <0.001 | 2.49 (1.56–3.99) | <0.001 |
| Low-risk source | 130 (49.2) | 25 (21.2) | 0.33 (0.21–0.51) | <0.001 | ||
| Baseline illness severity | ||||||
| Pitt score, m (IQR) | 1 (0–2) | 4 (1–4) | 1.38 (1.28–1.49) | <0.001 | 1.25 (1.13–1.37) | <0.001 |
| Pitt score ≥ 2 | 120 (45.5) | 86 (72.9) | 2.73 (1.82–4.1) | <0.001 | ||
| Septic shock | 51 (19.3) | 52 (44.1) | 2.63 (1.83–3.79) | <0.001 | 1.77 (1.18–2.65) | 0.006 |
| ICU admission | 44 (16.7) | 52 (44.1) | 2.99 (2.08–4.3) | <0.001 | ||
| Antibiotic management | ||||||
| Appropriate empirical treatment | 114 (43.2) | 50 (42.4) | 0.98 (0.68–1.41) | 0.899 | ||
| Appropriate definitive treatment | 240 (90.9) | 92 (78) | 0.42 (0.27–0.65) | <0.001 | 0.42 (0.27–0.66) | <0.001 |
| Combined antimicrobial therapy | 96 (36.4) | 54 (45.8) | 1.29 (0.9–1.87) | 0.157 | ||
| XDR PA BSI | 81 (30.7) | 41 (34.7) | 1.13 (0.77–1.65) | 0.535 | 1.14 (0.77–1.69) | 0.504 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, M.M.; López Montesinos, I.; Knobel, H.; Molas, E.; Sorlí, L.; Siverio-Parés, A.; Prim, N.; Segura, C.; Duran-Jordà, X.; Grau, S.; et al. Risk Factors for Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: What Is the Influence of XDR Phenotype on Outcomes? J. Clin. Med. 2020, 9, 514. https://doi.org/10.3390/jcm9020514
Montero MM, López Montesinos I, Knobel H, Molas E, Sorlí L, Siverio-Parés A, Prim N, Segura C, Duran-Jordà X, Grau S, et al. Risk Factors for Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: What Is the Influence of XDR Phenotype on Outcomes? Journal of Clinical Medicine. 2020; 9(2):514. https://doi.org/10.3390/jcm9020514
Chicago/Turabian StyleMontero, María Milagro, Inmaculada López Montesinos, Hernando Knobel, Ema Molas, Luisa Sorlí, Ana Siverio-Parés, Nuria Prim, Concepción Segura, Xavier Duran-Jordà, Santiago Grau, and et al. 2020. "Risk Factors for Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: What Is the Influence of XDR Phenotype on Outcomes?" Journal of Clinical Medicine 9, no. 2: 514. https://doi.org/10.3390/jcm9020514
APA StyleMontero, M. M., López Montesinos, I., Knobel, H., Molas, E., Sorlí, L., Siverio-Parés, A., Prim, N., Segura, C., Duran-Jordà, X., Grau, S., & Horcajada, J. P. (2020). Risk Factors for Mortality among Patients with Pseudomonas aeruginosa Bloodstream Infections: What Is the Influence of XDR Phenotype on Outcomes? Journal of Clinical Medicine, 9(2), 514. https://doi.org/10.3390/jcm9020514








