Microinvasive Fungal Rhinosinusitis: Proposal of a New Subtype in the Classification
Abstract
:1. Introduction
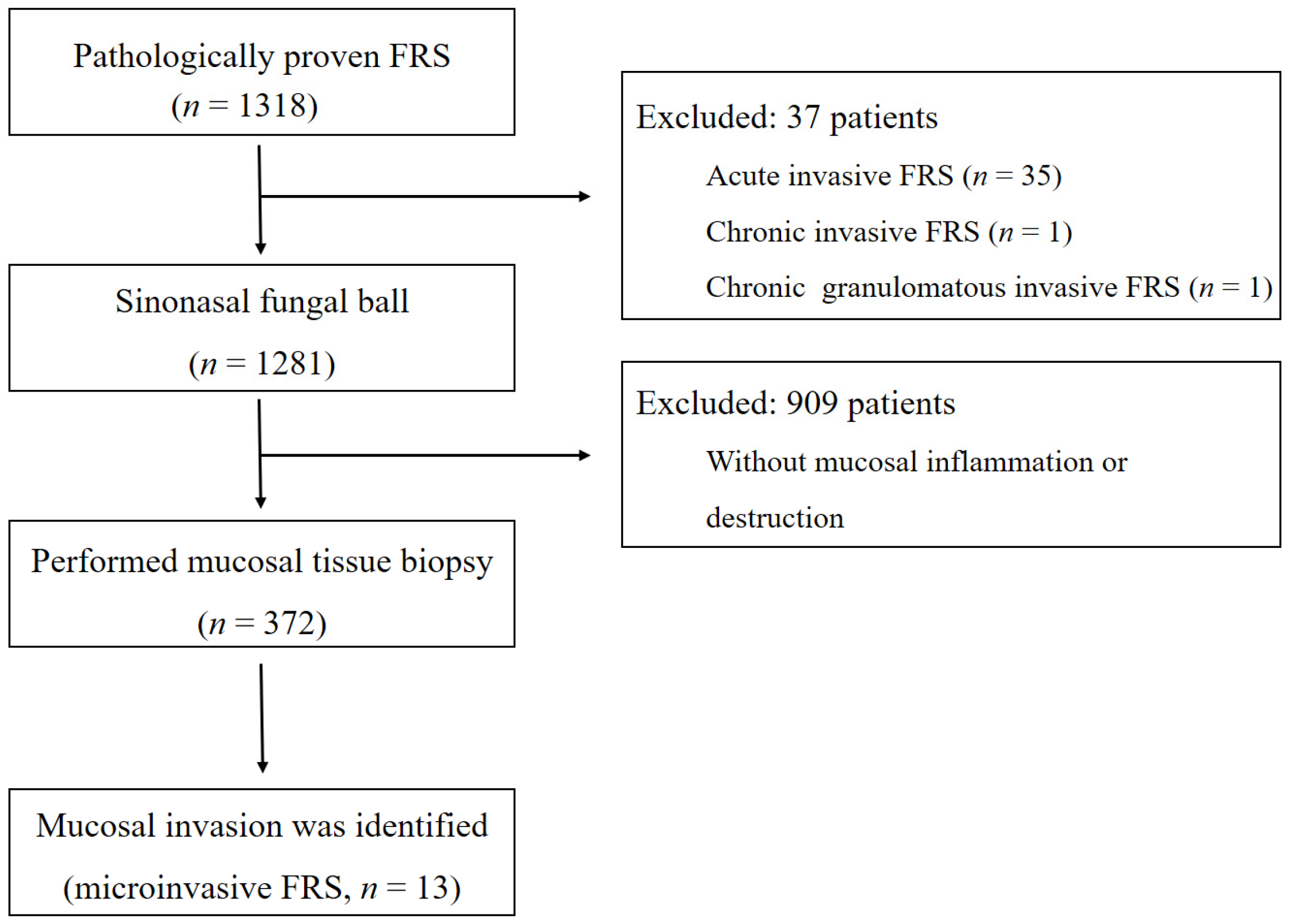
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hora, J.F. Primary Aspergillosis of the Paranasal Sinuses and Associated Areas. Laryngoscope 1965, 75, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.J.; Mitzner, R.; Kunchala, S.; Roland, L.; McGinn, J.D. Acute Invasive Fungal Rhinosinusitis: A 15-Year Experience with 41 Patients. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2016, 154, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Bakhshaee, M.; Bojdi, A.; Allahyari, A.; Majidi, M.R.; Tavakol, S.; Najafzadeh, M.J.; Asghari, M. Acute invasive fungal rhinosinusitis: Our experience with 18 cases. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. (EUFOS) Affil. Ger. Soc. Oto-Rhino-Laryngol. Head Neck Surg. 2016, 273, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Jang, M.S.; Hong, S.D.; Chung, S.K.; Kim, H.Y.; Dhong, H.J. Prognostic factors for survival in patients with acute invasive fungal rhinosinusitis. Am. J. Rhinol. Allergy 2015, 29, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Foshee, J.; Luminais, C.; Casey, J.; Farag, A.; Prestipino, A.; Iloreta, A.M.; Nyquist, G.; Rosen, M. An evaluation of invasive fungal sinusitis outcomes with subsite analysis and use of frozen section analysis. Int. Forum Allergy Rhinol. 2016, 6, 807–811. [Google Scholar] [CrossRef]
- Gode, S.; Turhal, G.; Ozturk, K.; Aysel, A.; Midilli, R.; Karci, B. Acute invasive fungal rhinosinusitis: Survival analysis and the prognostic indicators. Am. J. Rhinol. Allergy 2015, 29, e164–e169. [Google Scholar] [CrossRef]
- D’Anza, B.; Stokken, J.; Greene, J.S.; Kennedy, T.; Woodard, T.D.; Sindwani, R. Chronic invasive fungal sinusitis: Characterization and shift in management of a rare disease. Int. Forum Allergy Rhinol. 2016, 6, 1294–1300. [Google Scholar] [CrossRef]
- Parikh, S.L.; Venkatraman, G.; DelGaudio, J.M. Invasive fungal sinusitis: A 15-year review from a single institution. Am. J. Rhinol. 2004, 18, 75–81. [Google Scholar] [CrossRef]
- Grosjean, P.; Weber, R. Fungus balls of the paranasal sinuses: A review. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. (EUFOS) Affil. Ger. Soc. Oto-Rhino-Laryngol. Head Neck Surg. 2007, 264, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Patterson, T.F. Fungal disease of the nose and paranasal sinuses. J. Allergy Clin. Immunol. 2012, 129, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, P.; Lombardi, D.; Tomenzoli, D.; Villaret, A.B.; Piccioni, M.; Mensi, M.; Maroldi, R. Fungus ball of the paranasal sinuses: Experience in 160 patients treated with endoscopic surgery. Laryngoscope 2009, 119, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Simpson, G.; Healy, G.B. Fulminant aspergillosis of the nose and paranasal sinuses: A new clinical entity. Laryngoscope 1980, 90, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Fungus balls of the paranasal sinuses. Otolaryngol. Clin. N. Am. 2000, 33, 389–398. [Google Scholar] [CrossRef]
- Gungor, A.; Adusumilli, V.; Corey, J.P. Fungal sinusitis: Progression of disease in immunosuppression—A case report. Ear Nose Throat J. 1998, 77, 215. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, H.J.; Kim, H.Y.; Cha, J.; Lee, J.Y.; Chung, S.K.; Dhong, H.J.; Song, M.; Kim, S.T. Extrasinonasal infiltrative process associated with a sinonasal fungus ball: Does it mean invasive fungal sinusitis? Diagn. Interv. Radiol. (Ank. Turk.) 2016, 22, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Paknezhad, H.; Borchard, N.A.; Charville, G.W.; Ayoub, N.F.; Choby, G.W.; Thamboo, A.; Nayak, J.V. Evidence for a ‘preinvasive’ variant of fungal sinusitis: Tissue invasion without angioinvasion. World J. Otorhinolaryngol. Head Neck Surg. 2017, 3, 37–43. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Age (Years) | Sex | Location | Species | Underlying Disease | Symptoms | Duration of Symptoms (Months) | Antifungal Therapy | Disease-Free State at Last Visit | Duration of Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | F | (L) MS | Aspergillus | Facial pain | 1.5 | ND | Yes | 20 | |
| 2 | 82 | F | (R) MS | Aspergillus | DM | Nasal obstruction | 2 | Itraconazole 8 weeks | Yes | 21 |
| 3 | 83 | M | (L) SS | Aspergillus | DM | None | Voriconazole 17 weeks | Yes | 22 | |
| 4 | 63 | M | (L) MS | Aspergillus | Postnasal drip | 4 | ND | Yes | 22 | |
| 5 | 67 | F | (L) MS, ES | Aspergillus | DM | Headache | 120 | Amphotericin B 3 weeks, voriconazole 7 weeks & caspofungin 3 weeks | Yes | 23 |
| 6 | 48 | M | (R) SS | Aspergillus | Acute biphasic leukemia | None | Amphotericin B 8 weeks | Yes | 24 | |
| 7 | 64 | F | (R) MS, ES | Aspergillus | Purulent rhinorrhea | 1 | ND | Yes | 22 | |
| 8 | 72 | F | (R) MS, ES | Aspergillus | Postnasal drip | 12 | ND | Yes | 25 | |
| 9 | 62 | F | (L) MS | Aspergillus | Purulent rhinorrhea | 27 | ND | Yes | 43 | |
| 10 | 68 | M | (R) MS | Aspergillus | None | ND | Yes | 49 | ||
| 11 | 61 | F | (R) MS | Aspergillus | None | ND | Yes | 117 | ||
| 12 | 77 | F | (L) MS | Aspergillus | Diffuse large B cell lymphoma | None | Amphotericin B 2 weeks & voriconazole 15 weeks | Yes | 122 | |
| 13 | 71 | F | (B) SS | Aspergillus | Purulent rhinorrhea | 30 | ND | Yes | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.Y.; Seok, H.; Lee, S.H.; Choi, J.E.; Hong, S.D.; Chung, S.-K.; Peck, K.R.; Kim, H.Y. Microinvasive Fungal Rhinosinusitis: Proposal of a New Subtype in the Classification. J. Clin. Med. 2020, 9, 600. https://doi.org/10.3390/jcm9020600
Seo MY, Seok H, Lee SH, Choi JE, Hong SD, Chung S-K, Peck KR, Kim HY. Microinvasive Fungal Rhinosinusitis: Proposal of a New Subtype in the Classification. Journal of Clinical Medicine. 2020; 9(2):600. https://doi.org/10.3390/jcm9020600
Chicago/Turabian StyleSeo, Min Young, Hyeri Seok, Seung Hoon Lee, Ji Eun Choi, Sang Duk Hong, Seung-Kyu Chung, Kyong Ran Peck, and Hyo Yeol Kim. 2020. "Microinvasive Fungal Rhinosinusitis: Proposal of a New Subtype in the Classification" Journal of Clinical Medicine 9, no. 2: 600. https://doi.org/10.3390/jcm9020600
APA StyleSeo, M. Y., Seok, H., Lee, S. H., Choi, J. E., Hong, S. D., Chung, S.-K., Peck, K. R., & Kim, H. Y. (2020). Microinvasive Fungal Rhinosinusitis: Proposal of a New Subtype in the Classification. Journal of Clinical Medicine, 9(2), 600. https://doi.org/10.3390/jcm9020600






