Lymphadenectomy is Unnecessary for Pure Ground-Glass Opacity Pulmonary Nodules
Abstract
1. Introduction
2. Experimental Section
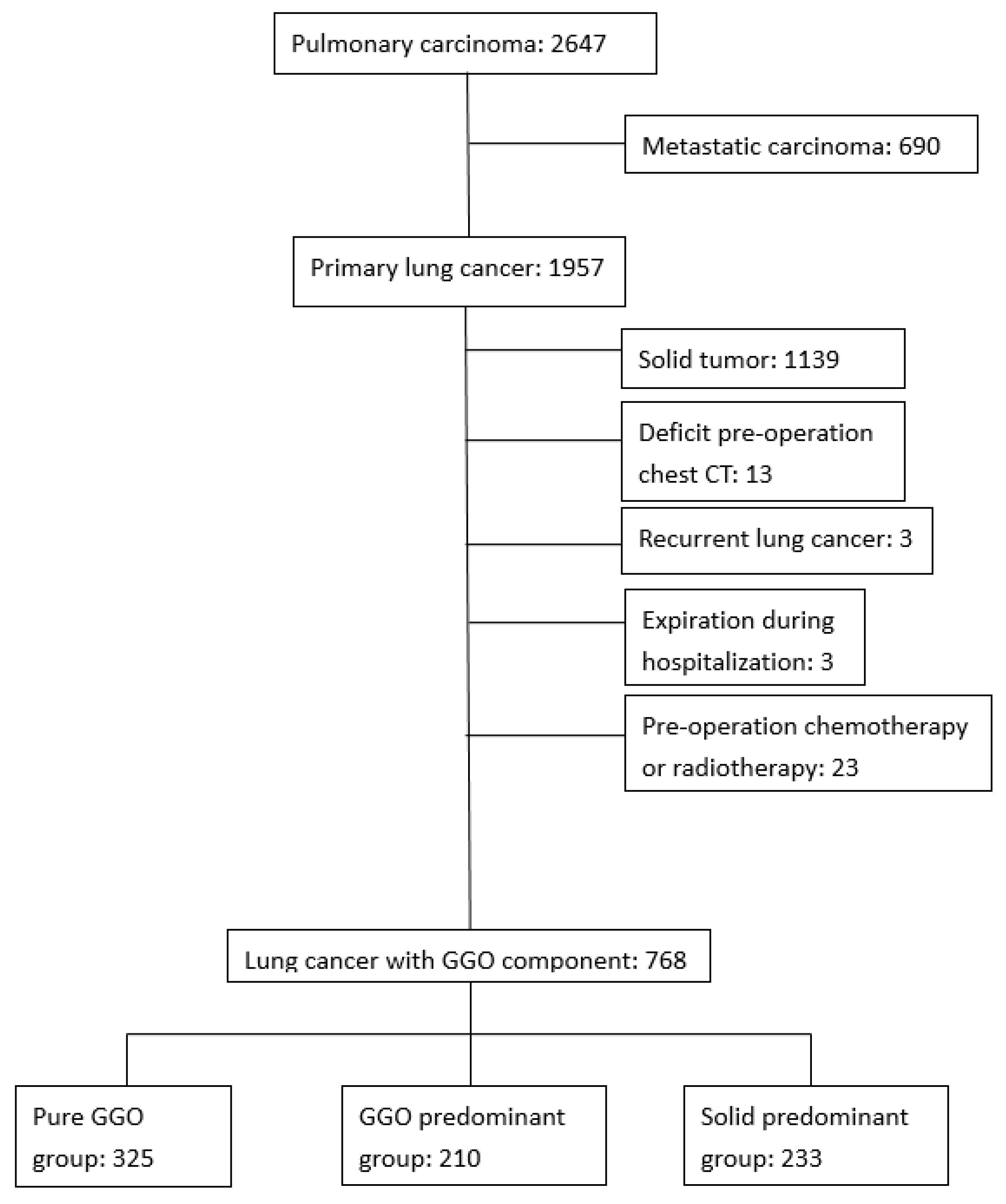
2.1. Data Source
2.2. Radiography
2.3. Participants
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Characteristics of GGO Patients with Lobectomy Resection
3.3. Multiple Cox Regression of GGO Patients with Lobectomy Resection
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Non-small Cell Lung Cancer (Version 2. 2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 21 November 2019).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. Cancer Treat. Res. 2016, 170, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Seidelman, J.L.; Myers, J.L.; Quint, L.E. Incidental, subsolid pulmonary nodules at CT: Etiology and management. Cancer Imaging 2013, 13, 365–373. [Google Scholar] [CrossRef]
- Hattori, A.; Matsunaga, T.; Hayashi, T.; Takamochi, K.; Oh, S.; Suzuki, K. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 954–962. [Google Scholar] [CrossRef]
- Hattori, A.; Matsunaga, T.; Takamochi, K.; Oh, S.; Suzuki, K. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann. Thorac. Surg. 2017, 104, 313–320. [Google Scholar] [CrossRef]
- Walter, J.E.; Heuvelmans, M.A.; Yousaf-Khan, U.; Dorrius, M.D.; Thunnissen, E.; Schermann, A.; Groen, H.J.M.; van der Aalst, C.M.; Nackaerts, K.; Vliegenthart, R.; et al. New Subsolid Pulmonary Nodules in Lung Cancer Screening: The NELSON Trial. J. Thorac. Oncol. 2018, 13, 1410–1414. [Google Scholar] [CrossRef]
- Matsuguma, H.; Yokoi, K.; Anraku, M.; Kondo, T.; Kamiyama, Y.; Mori, K.; Tominaga, K.; Tsuura, Y.; Honjo, S. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J. Thorac. Cardiovasc. Surg. 2002, 124, 278–284. [Google Scholar] [CrossRef]
- McLean, A.E.B.; Barnes, D.J.; Troy, L.K. Diagnosing Lung Cancer: The Complexities of Obtaining a Tissue Diagnosis in the Era of Minimally Invasive and Personalised Medicine. J. Clin. Med. 2018, 7, 163. [Google Scholar] [CrossRef]
- Fan, J.; Wang, L.; Jiang, G.N.; Gao, W. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann. Surg. Oncol. 2012, 19, 661–668. [Google Scholar] [CrossRef]
- Altorki, N.K.; Yip, R.; Hanaoka, T.; Bauer, T.; Aye, R.; Kohman, L.; Sheppard, B.; Thurer, R.; Andaz, S.; Smith, M.; et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J. Thorac. Cardiovasc. Surg. 2014, 147, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Doddoli, C.; Aragon, A.; Barlesi, F.; Chetaille, B.; Robitail, S.; Giudicelli, R.; Fuentes, P.; Thomas, P. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur. J. Cardiothorac. Surg. 2005, 27, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, J.; He, Z.; Yuan, P.; Huang, S.; Lv, W.; Hu, J. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer ≤2 cm. J. Thorac. Cardiovasc. Surg. 2018, 156, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Gajra, A.; Newman, N.; Gamble, G.P.; Kohman, L.J.; Graziano, S.L. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J. Clin. Oncol. 2003, 21, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H.; Zell, J.A. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 880–886. [Google Scholar] [CrossRef]
- Flores, R.M.; Nicastri, D.; Bauer, T.; Aye, R.; Andaz, S.; Kohman, L.; Sheppard, B.; Mayfield, W.; Thurer, R.; Korst, R.; et al. Computed Tomography Screening for Lung Cancer: Mediastinal Lymph Node Resection in Stage IA Nonsmall Cell Lung Cancer Manifesting as Subsolid and Solid Nodules. Ann. Surg. 2017, 265, 1025–1033. [Google Scholar] [CrossRef]
- Naruke, T.; Goya, T.; Tsuchiya, R.; Suemasu, K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann. Thorac. Surg. 1988, 46, 603–610. [Google Scholar] [CrossRef]
- Moon, Y.; Sung, S.W.; Moon, S.W.; Park, J.K. Risk factors for recurrence after sublobar resection in patients with small (2 cm or less) non-small cell lung cancer presenting as a solid-predominant tumor on chest computed tomography. J. Thorac. Dis. 2016, 8, 2018–2026. [Google Scholar] [CrossRef]
- Suzuki, K.; Kusumoto, M.; Watanabe, S.; Tsuchiya, R.; Asamura, H. Radiologic classification of small adenocarcinoma of the lung: Radiologic-pathologic correlation and its prognostic impact. Ann. Thorac. Surg. 2006, 81, 413–419. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Naruke, T.; Tsuchiya, R.; Kondo, H.; Nakayama, H.; Asamura, H. Lymph node sampling in lung cancer: How should it be done? Eur. J. Cardiothorac. Surg. 1999, 16, S17–S24. [Google Scholar] [CrossRef]
- Manser, R.; Wright, G.; Hart, D.; Byrnes, G.; Campbell, D.A. Surgery for early stage non-small cell lung cancer. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Wright, G.; Manser, R.L.; Byrnes, G.; Hart, D.; Campbell, D.A. Surgery for non-small cell lung cancer: Systematic review and meta-analysis of randomised controlled trials. Thorax 2006, 61, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Speicher, P.J.; Gu, L.; Gulack, B.C.; Wang, X.; D’Amico, T.A.; Hartwig, M.G.; Berry, M.F. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin. Lung Cancer 2016, 17, 47–55. [Google Scholar] [CrossRef]
- Haruki, T.; Aokage, K.; Miyoshi, T.; Hishida, T.; Ishii, G.; Yoshida, J.; Tsuboi, M.; Nakamura, H.; Nagai, K. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: Possibility of rational lymph node dissection. J. Thorac. Oncol. 2015, 10, 930–936. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Ojha, B.; Bryant, A.S.; Raghuveer, V.; Mountz, J.M.; Bartolucci, A.A. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann. Thorac. Surg. 2004, 78, 1017–1023. [Google Scholar] [CrossRef]
- Aoki, T. Growth of pure ground-glass lung nodule detected at computed tomography. J. Thorac. Dis. 2015, 7, E326–E328. [Google Scholar] [CrossRef]
- Nwogu, C.E.; Groman, A.; Fahey, D.; Yendamuri, S.; Dexter, E.; Demmy, T.L.; Miller, A.; Reid, M. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann. Thorac. Surg. 2012, 93, 1614–1619. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]

| Pure GGO (325) | GGO Predominant (210) | Solid Predominant (233) | P-Value | |
|---|---|---|---|---|
| Age (Year) | 57.2 ± 10.4 | 62.1 ± 9.8 | 64.4 ± 10.1 | <0.001 |
| Gender (%) | 0.057 | |||
| Male | 103 (31.7) | 90 (42.9) | 102 (43.8) | |
| Female | 212 (65.2) | 120 (57.1) | 131 (56.2) | |
| CPD (%) | 126 (38.8) | 90 (42.9) | 109 (46.8) | 0.166 |
| Smoking (%) | 52 (16.0) | 33 (15.7) | 50 (21.5) | 0.176 |
| Family history (%) | 69 (21.2) | 21 (10.0) | 17 (7.3) | <0.001 |
| Location (%) | 0.943 | |||
| Peripheral | 221 (68%) | 141 (67.1%) | 160 (68.7%) | |
| Central | 104 (32%) | 69 (32.9%) | 73 (31.3%) | |
| Tumor size (cm) | ||||
| GGO part | 1.1 ± 0.5 | 1.9 ± 0.8 | 2.2 ± 0.9 | <0.001 |
| Solid part | 0 | 0.5 ± 0.4 | 1.6 ± 0.7 | <0.001 |
| Clinical N stage (%) | 0.344 | |||
| 0 | 307 (94.5) | 206 (98.1) | 222 (94.8) | |
| 1 | 14 (4.3) | 0 | 3 (1.4) | |
| 2 | 4 (1.2) | 4 (1.9) | 8 (3.8) | |
| Operative method (%) | <0.001 | |||
| Sublobar resection | 234 (72.0) | 99 (47.1) | 64 (27.5) | |
| Lobectomy/bilobectomy | 91 (28.0) | 121 (52.9) | 169 (72.5) | |
| Lymph node stations | 3.4 ± 1.6 | 4.3 ± 1.7 | 4.6 ± 1.5 | <0.001 |
| N2 stations | 2.3 ± 0.9 | 2.5 ± 0.9 | 2.6 ± 0.8 | 0.002 |
| N1 stations | 1.1 ± 1.1 | 1.8 ± 1.2 | 2.0 ± 1.1 | <0.001 |
| Lymph node number | 10.8 ± 7.8 | 13.3 ± 7.2 | 16.0 ± 8.8 | <0.001 |
| N2 number | 7.6 ± 6.2 | 8.1 ± 5.2 | 9.4 ± 6.4 | 0.003 |
| N1 number | 3.1 ± 3.9 | 5.2 ± 4.5 | 6.7 ± 4.9 | <0.001 |
| Histology (%) | <0.001 | |||
| AIS | 154 (47.4) | 21 (10.0) | 0 (0) | |
| MIA | 77 (23.7) | 21 (10.0) | 11 (4.7) | |
| LPA | 63 (19.4) | 72 (34.3) | 36 (15.5) | |
| IPA | 31 (9.5) | 94 (44.8) | 179 (76.8) | |
| Other cancer | 0 (0) | 2 (1.0) | 8 (3.4) | |
| T Stage (%) | <0.001 | |||
| 0 | 154 (47.4) | 21 (10.0) | 0 (0) | |
| 1a | 131 (40.3) | 91 (43.3) | 56 (24.0) | |
| 1b | 2 (0.6) | 16 (7.6) | 20 (8.6) | |
| 1c | 0 | 5 (2.4) | 11 (4.7) | |
| 2a | 38 (11.7) | 76 (36.2) | 144 (61.8) | |
| 4 | 0 (0) | 1 (0.5) | 2 (0.9) | |
| N stage (%) | 0.057 | |||
| 0 | 321 (98.8) | 198 (94.3) | 215 (92.3) | |
| 1 | 0 | 3 (1.4) | 6 (2.6) | |
| 2 | 0 | 5 (2.4) | 10 (4.3) | |
| No lymphadenectomy | 3 (1.2) | 4 (1.9) | 2 (0.9) | |
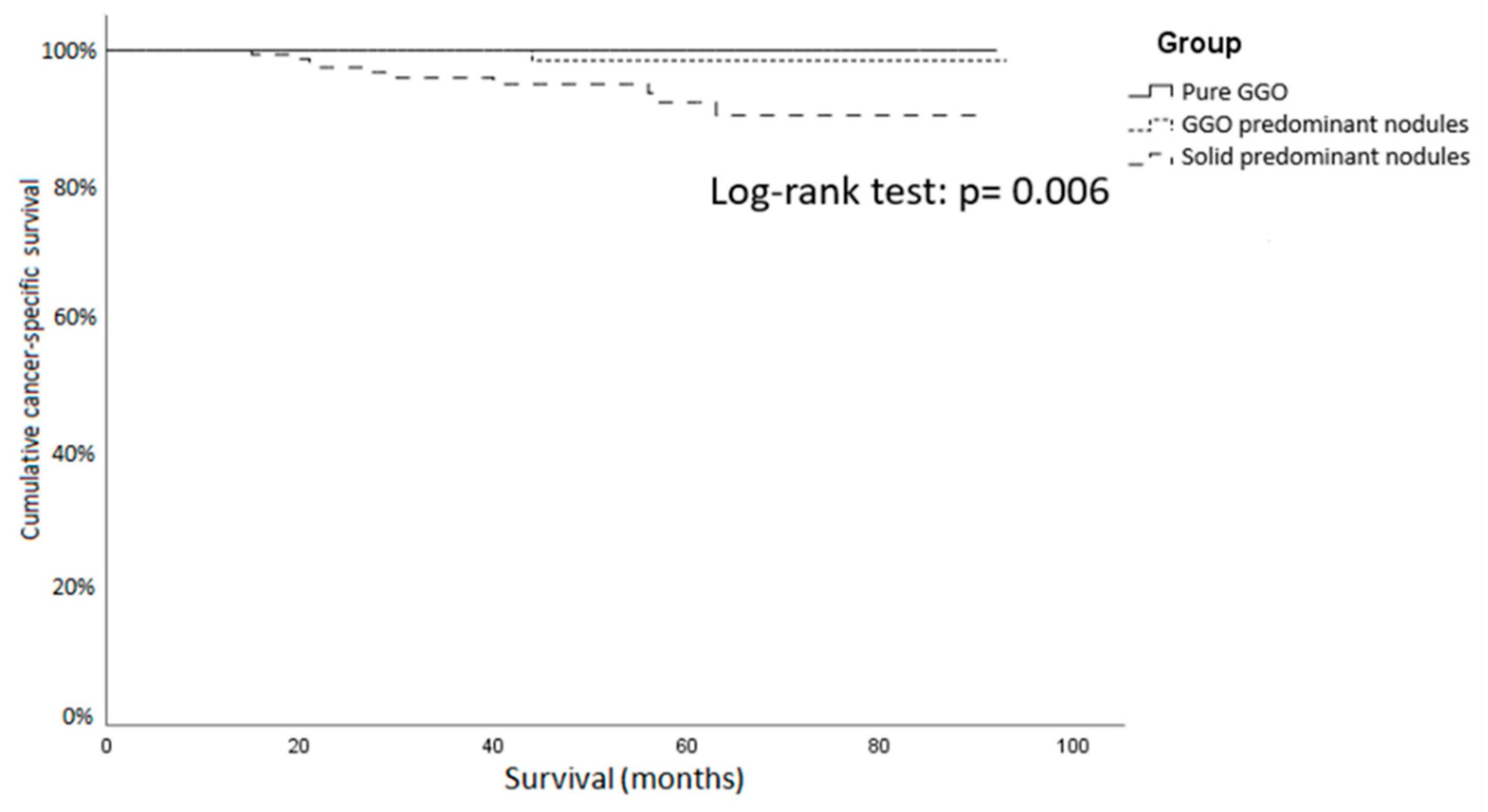
| Recurrence (%) | 0 | 5 (2.4) | 21 (9.0) | <0.001 |
| Disease-related death (%) | 0 | 3 (1.4) | 12 (5.2) | 0.013 |
| Pure GGO (91) | GGO Predominant (122) | Solid Predominant (169) | P-Value | |
|---|---|---|---|---|
| Age (Year) | 57.9 ± 9.0 | 60.2 ± 9.9 | 63.5 ± 9.5 | <0.001 |
| Gender (%) | 0.276 | |||
| Male | 29 (31.9) | 51 (41.8) | 69 (40.8) | |
| Female | 62 (68.1) | 71 (58.2) | 100 (59.2) | |
| CPD (%) | 31 (34.1) | 47 (35.8) | 69 (40.8) | 0.567 |
| Smoking (%) | 19 (20.9) | 19 (15.6) | 33 (19.5) | 0.566 |
| Family history (%) | 19 (20.9) | 12 (9.8) | 12 (7.1) | 0.003 |
| Location (%) | 0.743 | |||
| Peripheral | 55 (60.4) | 76 (62.3) | 110 (65.1) | |
| Central | 36 (39.6) | 46 (37.7) | 59 (34.9) | |
| Tumor size (cm) | ||||
| GGO part | 1.4 ± 0.6 | 2.1 ± 0.9 | 2.4 ± 0.9 | <0.001 |
| Solid part | 0 | 0.6 ± 0.4 | 1.7 ± 0.7 | <0.001 |
| Clinical N stage (%) | 0.458 | |||
| 0 | 83 (91.2) | 119 (97.5) | 161 (95.2) | |
| 1 | 6 (6.6) | 0 | 3 (1.8) | |
| 2 | 2 (2.2) | 3 (2.5) | 5 (3.0) | |
| Lymph node number | 17.0 ± 8.0 | 15.7 ± 5.8 | 18.3 ± 8.3 | 0.014 |
| N2 number | 10.0 ± 7.1 | 8.3 ± 4.5 | 10.2 ± 6.8 | 0.031 |
| N1 number | 6.9 ± 3.6 | 7.4 ± 3.8 | 8.1 ± 4.4 | 0.049 |
| Histology (%) | <0.001 | |||
| AIS | 28 (30.8) | 4 (3.2) | 0 | |
| MIA | 20 (22.0) | 10 (8.2) | 3 (1.8) | |
| LPA | 28 (30.8) | 45 (36.9) | 25 (14.8) | |
| IPA | 15 (16.5) | 63 (51.6) | 136 (80.5) | |
| Other cancer | 0 | 0 | 5 (3.0) | |
| T Stage (%) | <0.001 | |||
| 0 | 28 (30.8) | 4 (3.3) | 0 | |
| 1a | 46 (50.5) | 48 (39.3) | 29 (17.2) | |
| 1b | 1 (1.1) | 10 (8.2) | 15 (8.9) | |
| 1c | 0 | 5 (4.1) | 11 (6.5) | |
| 2a | 16 (17.6) | 54 (44.3) | 113 (66.9) | |
| 4 | 0 | 1 (0.8) | 1 (0.6) | |
| N stage (%) | 0.041 | |||
| 0 | 91 (100) | 116 (95.1) | 155 (91.7) | |
| 1 | 0 | 3 (2.5) | 6 (3.6) | |
| 2 | 0 | 3 (2.5) | 8 (4.7) | |
| Recurrence (%) | 0 | 1 (0.8) | 17 (10.1) | <0.001 |
| Disease-related death (%) | 0 | 1 (0.8) | 10 (5.9) | 0.006 |
| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | |||
| ≤60 years | - | ||
| >60 years | 0.622 | 0.259–1.492 | 0.287 |
| Family History | |||
| No | - | ||
| Yes | 1.003 | 0.231–4.366 | 0.996 |
| GGO property | |||
| Pure GGO | - | ||
| GGO predominant | 4.842 | 0.576–40.707 | 0.146 |
| Solid predominant part solid GGO | 8.197 | 1.052–63.862 | 0.045 |
| Lymph node numbers | |||
| ≤15 | - | ||
| >15 | 3.564 | 1.193–10.648 | 0.023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chen, C.-K.; Hsieh, C.-C.; Hsu, W.-H.; Wu, Y.-C.; Hung, J.-J.; Hsu, P.-K.; Hsu, H.-S. Lymphadenectomy is Unnecessary for Pure Ground-Glass Opacity Pulmonary Nodules. J. Clin. Med. 2020, 9, 672. https://doi.org/10.3390/jcm9030672
Lin Y-H, Chen C-K, Hsieh C-C, Hsu W-H, Wu Y-C, Hung J-J, Hsu P-K, Hsu H-S. Lymphadenectomy is Unnecessary for Pure Ground-Glass Opacity Pulmonary Nodules. Journal of Clinical Medicine. 2020; 9(3):672. https://doi.org/10.3390/jcm9030672
Chicago/Turabian StyleLin, Yi-Han, Chun-Ku Chen, Chih-Cheng Hsieh, Wen-Hu Hsu, Yu-Chung Wu, Jung-Jyh Hung, Po-Kuei Hsu, and Han-Shui Hsu. 2020. "Lymphadenectomy is Unnecessary for Pure Ground-Glass Opacity Pulmonary Nodules" Journal of Clinical Medicine 9, no. 3: 672. https://doi.org/10.3390/jcm9030672
APA StyleLin, Y.-H., Chen, C.-K., Hsieh, C.-C., Hsu, W.-H., Wu, Y.-C., Hung, J.-J., Hsu, P.-K., & Hsu, H.-S. (2020). Lymphadenectomy is Unnecessary for Pure Ground-Glass Opacity Pulmonary Nodules. Journal of Clinical Medicine, 9(3), 672. https://doi.org/10.3390/jcm9030672





