Parafoveal and Peripapillary Perfusion Predict Visual Field Recovery in Chiasmal Compression due to Pituitary Tumors
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Danesh-Meyer, H.V.; Carroll, S.C.; Foroozan, R.; Savino, P.J.; Fan, J.; Jiang, Y.; Vander Hoorn, S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4827–4835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danesh-Meyer, H.V.; Papchenko, T.; Savino, P.J.; Law, A.; Evans, J.; Gamble, G.D. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1879–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.H.; Hwang, S.C.; Kim, B.T.; Ohn, Y.H.; Park, T.K. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8527–8533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, T.; Sanchez, S.; Litre, C.F.; Radoi, C.; Delemer, B.; Rousseaux, P.; Ducasse, A.; Arndt, C. Prognostic value of retinal nerve fiber layer thickness for postoperative peripheral visual field recovery in optic chiasm compression. J. Neurosurg. 2014, 121, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Raverot, G.; Jouanneau, E.; Borson-Chazot, F.; Perrin, G.; Rabilloud, M.; Tilikete, C.; Bernard, M.; Vighetto, A. Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am. J. Ophthalmol. 2009, 147, 64–70.e62. [Google Scholar] [CrossRef]
- Barzaghi, L.R.; Medone, M.; Losa, M.; Bianchi, S.; Giovanelli, M.; Mortini, P. Prognostic factors of visual field improvement after trans-sphenoidal approach for pituitary macroadenomas: Review of the literature and analysis by quantitative method. Neurosurg. Rev. 2012, 35, 369–379. [Google Scholar] [CrossRef]
- Yu, F.F.; Chen, L.L.; Su, Y.H.; Huo, L.H.; Lin, X.X.; Liao, R.D. Factors influencing improvement of visual field after trans-sphenoidal resection of pituitary macroadenomas: A retrospective cohort study. Int. J. Ophthalmol. 2015, 8, 1224–1228. [Google Scholar]
- Ohkubo, S.; Higashide, T.; Takeda, H.; Murotani, E.; Hayashi, Y.; Sugiyama, K. Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn. J. Ophthalmol. 2012, 56, 68–75. [Google Scholar] [CrossRef]
- Monteiro, M.L.; Hokazono, K.; Fernandes, D.B.; Costa-Cunha, L.V.; Sousa, R.M.; Raza, A.S.; Wang, D.L.; Hood, D.C. Evaluation of inner retinal layers in eyes with temporal hemianopic visual loss from chiasmal compression using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3328–3336. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.; Medeiros, F.A.; Weinreb, R.N. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016, 123, 2498–2508. [Google Scholar] [CrossRef]
- Gaier, E.D.; Wang, M.; Gilbert, A.L.; Rizzo, J.F., 3rd; Cestari, D.M.; Miller, J.B. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PLoS ONE 2018, 13, e0199793. [Google Scholar] [CrossRef] [PubMed]
- Kwapong, W.R.; Peng, C.; He, Z.; Zhuang, X.; Shen, M.; Lu, F. Altered Macular Microvasculature in Neuromyelitis Optica Spectrum Disorders. Am. J. Ophthalmol. 2018, 192, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Soetikno, B.T.; Fawzi, A.A. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina 2016, 36, 2039–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sheikh, M.; Tepelus, T.C.; Nazikyan, T.; Sadda, S.R. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 449–452. [Google Scholar] [CrossRef]
- Forte, R.; Haulani, H.; Dyrda, A.; Jurgens, I. Swept source optical coherence tomography angiography in patients treated with hydroxychloroquine: Correlation with morphological and functional tests. Br. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Ghahari, E.; Bowd, C.; Zangwill, L.M.; Proudfoot, J.; Hasenstab, K.A.; Hou, H.; Penteado, R.C.; Manalastas, P.I.C.; Moghimi, S.; Shoji, T.; et al. Association of Macular and Circumpapillary Microvasculature with Visual Field Sensitivity in Advanced Glaucoma. Am. J. Ophthalmol. 2019, 204, 51–61. [Google Scholar] [CrossRef]
- Higashiyama, T.; Ichiyama, Y.; Muraki, S.; Nishida, Y.; Ohji, M. Optical Coherence Tomography Angiography of Retinal Perfusion in Chiasmal Compression. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 724–729. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Tian, J.J.; Akil, H.; Garcia, G.A.; Sadda, S.R.; Sadun, A.A. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina 2016, 36, S168–S177. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Spain, R.; Potsaid, B.; Liu, J.J.; Baumann, B.; Hornegger, J.; Fujimoto, J.G.; Wu, Q.; Huang, D. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br. J. Ophthalmol. 2014, 98, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Feucht, N.; Maier, M.; Lepennetier, G.; Pettenkofer, M.; Wetzlmair, C.; Daltrozzo, T.; Scherm, P.; Zimmer, C.; Hoshi, M.M.; Hemmer, B.; et al. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult. Scler. 2019, 25, 224–234. [Google Scholar] [CrossRef]
- Lee, G.I.; Park, K.A.; Oh, S.Y.; Kong, D.S. Analysis of Optic Chiasmal Compression Caused by Brain Tumors Using Optical Coherence Tomography Angiography. Sci. Rep. 2020, 10, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanalingham, K.K.; Bhattacharjee, S.; Pennington, R.; Ng, J.; Mendoza, N. The time course of visual field recovery following transphenoidal surgery for pituitary adenomas: Predictive factors for a good outcome. J. Neurol. Neurosurg. Psychiatry 2005, 76, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneoka, Y.; Hatase, T.; Watanabe, N.; Jinguji, S.; Okada, M.; Takagi, M.; Fujii, Y. Early morphological recovery of the optic chiasm is associated with excellent visual outcome in patients with compressive chiasmal syndrome caused by pituitary tumors. Neurol. Res. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.J.; Yu, Y.S.; Kim, Y.H.; Paek, S.H.; Kim, D.G.; Jung, H.W. Prognostic factors for visual recovery after transsphenoidal pituitary adenectomy. Br. J. Neurosurg. 2013, 27, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Meyer, H.V.; Wong, A.; Papchenko, T.; Matheos, K.; Stylli, S.; Nichols, A.; Frampton, C.; Daniell, M.; Savino, P.J.; Kaye, A.H. Optical coherence tomography predicts visual outcome for pituitary tumors. J. Clin. Neurosci. 2015, 22, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Oh, M.C.; Kim, E.H.; Kim, C.Y.; Kim, S.H.; Lee, K.S.; Chang, J.H. Use of optical coherence tomography to predict visual outcome in parachiasmal meningioma. J. Neurosurg. 2015, 123, 1489–1499. [Google Scholar] [CrossRef] [Green Version]
- Blanch, R.J.; Micieli, J.A.; Oyesiku, N.M.; Newman, N.J.; Biousse, V. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary 2018, 21, 515–523. [Google Scholar] [CrossRef]
- Moon, C.H.; Hwang, S.C.; Ohn, Y.H.; Park, T.K. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7966–7973. [Google Scholar] [CrossRef]
- Yum, H.R.; Park, S.H.; Park, H.Y.; Shin, S.Y. Macular Ganglion Cell Analysis Determined by Cirrus HD Optical Coherence Tomography for Early Detecting Chiasmal Compression. PLoS ONE 2016, 11, e0153064. [Google Scholar] [CrossRef]
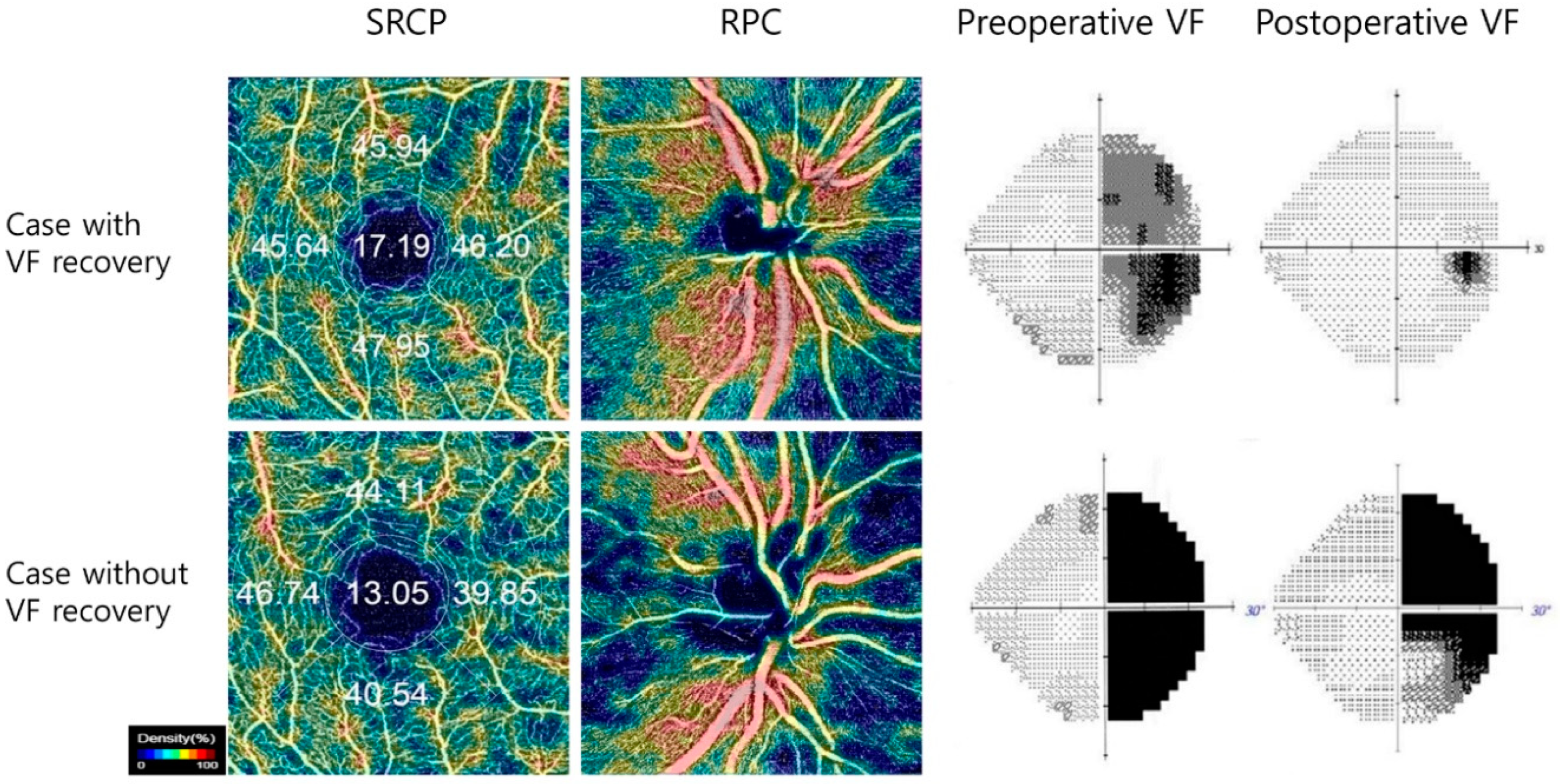
| Patients (n = 57) | Healthy Controls (n = 42) | p value | |
|---|---|---|---|
| Age | 49 ± 13 | 49 ± 11 | 0.749 * |
| Gender (M/F) | 22/35 | 17/25 | 1.000 † |
| Spherical equivalent | −1.14 ± 1.98 | −1.32 ± 1.84 | 0.316 ‡ |
| Symptom duration (mon) | 5 ± 11 | - | |
| Preoperative VF (MD) | −7.27 ± 6.20 | 0.07 ± 1.18 | <0.001 ‡ |
| Patients (n = 57) | Healthy Controls (n = 42) | p value * | |
|---|---|---|---|
| RNFL thickness (μm) | 90.76 ± 10.68 | 99.96 ± 8.30 | <0.001 |
| GCC thickness (μm) | 75.85 ± 8.37 | 83.77 ± 5.91 | <0.001 |
| Vessel densities of SRCP (%) | 47.46 ± 2.03 | 48.67 ± 2.39 | 0.007 |
| Vessel densities of DRCP (%) | 48.77 ± 2.16 | 49.42 ± 2.18 | 0.141 |
| Vessel densities of RPC (%) | 56.91 ± 3.41 | 58.41 ± 2.55 | 0.020 |
| Univariate Analysis | Estimate | Standard Error | p Value |
|---|---|---|---|
| Gender | 0.002 | 0.998 | 0.998 |
| Age | 0.005 | 0.031 | 0.863 |
| Spherical equivalent | −0.282 | 0.202 | 0.169 |
| Symptom duration | −0.066 | 0.039 | 0.093 |
| Preoperative VF defects | 0.194 | 0.06 | 0.002 |
| pRNFL thickness | |||
| Average | 0.059 | 0.037 | 0.122 |
| Temporal | 0.025 | 0.024 | 0.314 |
| Inferior | 0.034 | 0.025 | 0.167 |
| Nasal | 0.062 | 0.038 | 0.107 |
| Superior | 0.013 | 0.023 | 0.587 |
| GCC thickness | |||
| Average | 0.066 | 0.05 | 0.192 |
| Temporal | 0.019 | 0.063 | 0.765 |
| Inferior | 0.056 | 0.049 | 0.258 |
| Nasal | 0.060 | 0.038 | 0.123 |
| Superior | 0.078 | 0.04 | 0.058 |
| SRCP | |||
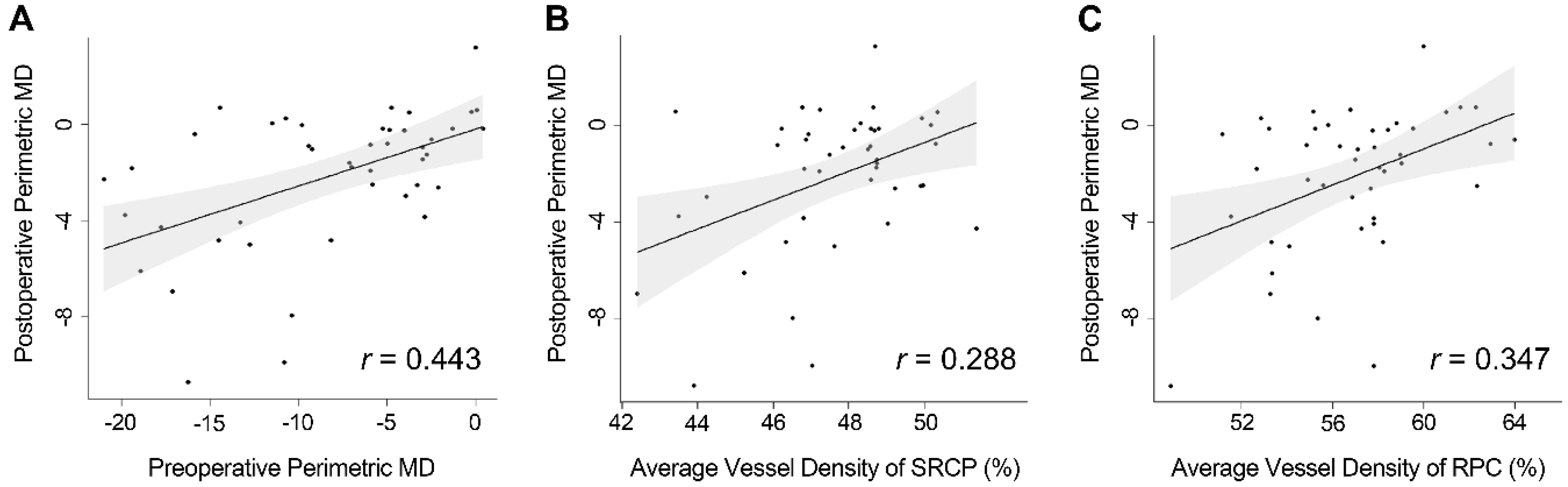
| Average | 0.468 | 0.19 | 0.017 |
| Temporal | 0.268 | 0.162 | 0.103 |
| Inferior | 0.232 | 0.126 | 0.072 |
| Nasal | 0.180 | 0.157 | 0.257 |
| Superior | 0.234 | 0.116 | 0.049 |
| DRCP | |||
| Average | 0.284 | 0.185 | 0.131 |
| Temporal | 0.123 | 0.141 | 0.386 |
| Inferior | 0.121 | 0.111 | 0.281 |
| Nasal | 0.159 | 0.130 | 0.227 |
| Superior | 0.109 | 0.125 | 0.388 |
| RPC | |||
| Average | 0.353 | 0.113 | 0.003 |
| Temporal | 0.142 | 0.082 | 0.088 |
| Inferior | 0.097 | 0.087 | 0.270 |
| Nasal | 0.129 | 0.064 | 0.051 |
| Superior | 0.138 | 0.063 | 0.032 |
| Model 1* | Model 2* | |||||
|---|---|---|---|---|---|---|
| Variables | Estimate | Standard Error | p Value | Estimate | Standard Error | p Value |
| Preoperative VF defects | 0.204 | 0.062 | 0.002 | 0.217 | 0.06 | 0.001 |
| Symptom duration | NS | NS | NS | NS | NS | NS |
| pRNFL thickness (nasal) | NS | NS | NS | NS | NS | NS |
| GCC thickness (superior) | NS | NS | NS | NS | NS | NS |
| RPC (average) (Model 1) | NS | NS | NS | - | - | - |
| RPC (superior) (Model 2) | - | - | - | NS | NS | NS |
| SRCP (average) (Model 1) | 0.444 | 0.191 | 0.025 | - | - | - |
| SRCP (superior) (Model 2) | - | - | - | 0.305 | 0.118 | 0.013 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-I.; Park, K.-A.; Oh, S.Y.; Kong, D.-S. Parafoveal and Peripapillary Perfusion Predict Visual Field Recovery in Chiasmal Compression due to Pituitary Tumors. J. Clin. Med. 2020, 9, 697. https://doi.org/10.3390/jcm9030697
Lee G-I, Park K-A, Oh SY, Kong D-S. Parafoveal and Peripapillary Perfusion Predict Visual Field Recovery in Chiasmal Compression due to Pituitary Tumors. Journal of Clinical Medicine. 2020; 9(3):697. https://doi.org/10.3390/jcm9030697
Chicago/Turabian StyleLee, Ga-In, Kyung-Ah Park, Sei Yeul Oh, and Doo-Sik Kong. 2020. "Parafoveal and Peripapillary Perfusion Predict Visual Field Recovery in Chiasmal Compression due to Pituitary Tumors" Journal of Clinical Medicine 9, no. 3: 697. https://doi.org/10.3390/jcm9030697
APA StyleLee, G.-I., Park, K.-A., Oh, S. Y., & Kong, D.-S. (2020). Parafoveal and Peripapillary Perfusion Predict Visual Field Recovery in Chiasmal Compression due to Pituitary Tumors. Journal of Clinical Medicine, 9(3), 697. https://doi.org/10.3390/jcm9030697




